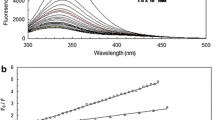
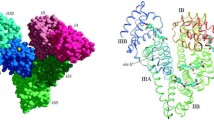
Abstract
Spectroscopic techniques have been used to improve the understanding of the interactions between hydroxyethyl starch (HES) 200/0.5 and human serum albumin (HSA) in a simulated physiological fluid of pH 7.4. It has been revealed that static fluorescence quenching occurred when HSA interacted with HES 200/0.5. The negative value of ΔH° (− 2.39 × 104 J·mol−1) and positive value of ΔS° (30.1 J·mol−1·K−1) suggested electrostatic interaction was the dominating force of the binding reaction between HES 200/0.5 to HSA, and the binding process was proved as a spontaneous one with negative ΔG° values (− 3.34 × 105 J·mol−1 at body temperature). The distance between HSA and HES 200/0.5 (r = 2.11 nm) proved efficient energy transfer from Trp to the drug. Moreover, the binding site of HES 200/0.5 to HSA was confirmed by site marker competitive experiments as Sudlow’s site I. Finally, it was observed that the conformation of HSA was changed with a loss of α-helical but acquisition of β contents, where a more stabilized secondary structure was formed.







Similar content being viewed by others
References
Zhao, X., Li, P., Song, S., Wang, H., Zhao, L., Zong, W., Zhang, H., Qu, G., Hu, L., Cai, Z.: Molecular structural heterogeneity of bisphenols governs their serum albumin binding. Sci. Total Environ. 781, 146499 (2021)
Goodman, D.S.: The interaction of human serum albumin with long-chain fatty acid anions. J. Am. Chem. Soc. 80, 3892–3898 (1958)
Koch-Weser, J., Sellers, E.M.: Binding of drugs to serum albumin. N. Engl. J. Med. 294, 311–316 (1976)
Sjöholm, I., Ekman, B., Kober, A., Ljungstedt-Påhlman, I., Seiving, B., Sjödin, T.: Binding of drugs to human serum albumin: XI. The specificity of three binding sites as studied with albumin immobilized in microparticles. Mol. Pharmacol. 16, 767–777 (1979)
He, X.M., Carter, D.C.: Atomic structure and chemistry of human serum albumin. Nature 358, 209 (1992)
Zhao, Z., Li, G., Liu, Q.S., Liu, W., Qu, G., Hu, L., Long, Y., Cai, Z., Zhao, X., Jiang, G.: Identification and interaction mechanism of protein corona on silver nanoparticles with different sizes and the cellular responses. J. Hazard. Mater. 414, 125582 (2021)
Nasrollahi, S., Ghoreishi, S.M., Ebrahimabadi, A.H., Khoobi, A.: Gas chromatography-mass spectrometry analysis and antimicrobial, antioxidant and anti-cancer activities of essential oils and extracts of Stachys schtschegleevii plant as biological macromolecules. Int. J. Biol. Macromol. 128, 718–723 (2019)
Ghoreishi, S.M., Behpour, M., Khoobi, A., Masoum, S.: Application of experimental design for quantification and voltammetric studies of sulfapyridine based on a nanostructure electrochemical sensor. Arab. J. Chem. 10, S3156–S3166 (2017)
Khoobi, A., Shahdost-fard, F., Arbabi, M., Akbari, M., Mirzaei, H., Nejati, M., Lotfinia, M., Sobhani-Nasab, A., Banafshe, H.R.: Sonochemical synthesis of ErVO4/MnWO4 heterostructures: application as a novel nanostructured surface for electrochemical determination of tyrosine in biological samples. Polyhedron 177, 114302 (2020)
Ghoreishi, S.M., Behpour, M., Khoobi, A., Moghadam, Z.: Determination of trace amounts of sulfamethizole using a multi-walled carbon nanotube modified electrode: application of experimental design in voltammetric studies. Anal. Lett. 46, 323–339 (2013)
Jungheinrich, C., Neff, T.A.: Pharmacokinetics of hydroxyethyl starch. Clin. Pharmacokinet. 44, 681–699 (2005)
Wiedermann, C.J., Joannidis, M.: Accumulation of hydroxyethyl starch in human and animal tissues: a systematic review. Intens. Care Med. 40, 160–170 (2014)
Kuitunen, A., Suojaranta-Ylinen, R., Kukkonen, S., Niemi, T.: A comparison of the haemodynamic effects of 4% succinylated gelatin, 6% hydroxyethyl starch (200/0.5) and 4% human albumin after cardiac surgery. Scand. J. Surg. 96, 72–78 (2007)
Vogt, N.H., Bothner, U., Lerch, G., Lindner, K.H., Georgieff, M.: Large-dose administration of 6% hydroxyethyl starch 200/0.5 for total hip arthroplasty: plasma homeostasis, hemostasis, and renal function compared to use of 5% human albumin. Anesthesia Analgesia 83, 262–268 (1996)
Song, S., Li, Y., Liu, Q.S., Wang, H., Li, P., Shi, J., Hu, L., Zhang, H., Liu, Y., Li, K.: Interaction of mercury ion (Hg2+) with blood and cytotoxicity attenuation by serum albumin binding. J. Hazard. Mater. 412, 125158 (2021)
Rahnama, E., Mahmoodian-Moghaddam, M., Khorsand-Ahmadi, S., Saberi, M.R., Chamani, J.: Binding site identification of metformin to human serum albumin and glycated human serum albumin by spectroscopic and molecular modeling techniques: a comparison study. J. Biomol. Struct. Dyn. 33, 513–533 (2015)
Chanphai, P., Tajmir-Riahi, H.: Tea polyphenols bind serum albumins: a potential application for polyphenol delivery. Food Hydrocolloids 89, 461–467 (2019)
Xu, C., Zhao, X., Wang, L., Zhang, X., Wang, Y., Lan, J.: Protein conjugation with gold nanoparticles: spectroscopic and thermodynamic analysis on the conformational and activity of serum albumin. J. Nanosci. Nanotechnol. 18, 7818–7823 (2018)
Chanphai, P., Ouellette, V., Bérubé, G., Tajmir-Riahi, H.: Conjugation of testo and testo-Pt (II) with serum proteins: loading efficacy and protein conformation. Int. J. Biol. Macromol. 118, 1112–1119 (2018)
Soltanabadi, O., Atri, M.S., Bagheri, M.: Spectroscopic analysis, docking and molecular dynamics simulation of the interaction of cinnamaldehyde with human serum albumin. J. Incl. Phenom. Macrocycl. Chem. 91, 189–197 (2018)
Zhao, X., Lu, D., Liu, Q.S., Li, Y., Feng, R., Hao, F., Qu, G., Zhou, Q., Jiang, G.: Hematological effects of gold nanorods on erythrocytes: hemolysis and hemoglobin conformational and functional changes. Adv. Sci. 4, 1700296 (2017)
Thakur, R., Das, A., Sharma, V., Adhikari, C., Ghosh, K.S., Chakraborty, A.: Interaction of different prototropic species of an anticancer drug ellipticine with HSA and IgG proteins: multispectroscopic and molecular modeling studies. Phys. Chem. Chem. Phys. 17, 16937–16946 (2015)
Zhao, X.C., Liu, R.T., Chi, Z.X., Teng, Y., Qin, P.F.: New insights into the behavior of bovine serum albumin adsorbed onto carbon nanotubes: comprehensive spectroscopic studies. J. Phys. Chem. B 114, 5625–5631 (2010)
Zhao, X., Lu, D., Hao, F., Liu, R.: Exploring the diameter and surface dependent conformational changes in carbon nanotube-protein corona and the related cytotoxicity. J. Hazard. Mater. 292, 98–107 (2015)
Zhu, Y., Zhang, R., Wang, Y., Ma, J., Li, K., Li, Z.: Biophysical study on the interaction of an anesthetic, vecuronium bromide with human serum albumin using spectroscopic and calorimetric methods. J. Photochem. Photobiol. B 140, 381–389 (2014)
Bourassa, P., Hasni, I., Tajmir-Riahi, H.A.: Folic acid complexes with human and bovine serum albumins. Food Chem. 129, 1148–1155 (2011)
Zhao, X., Hao, F., Lu, D., Liu, W., Zhou, Q., Jiang, G.: Influence of the surface functional group density on the carbon-nanotube-induced α-chymotrypsin structure and activity alterations. ACS Appl. Mater. Interfaces 7, 18880–18890 (2015)
Sneharani, A.H., Karakkat, J.V., Singh, S.A., Rao, A.G.A.: Interaction of curcumin with beta-lactoglobulin-stability, spectroscopic analysis, and molecular modeling of the complex. J. Agric. Food Chem. 58, 11130–11139 (2010)
Ray, A., Seth, B.K., Pal, U., Basu, S.: Nickel(II)-Schiff base complex recognizing domain II of bovine and human serum albumin: spectroscopic and docking studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 92, 164–174 (2012)
Varlan, A., Ionescu, S., Hillebrand, M.: Study of the interaction between ofloxacin and human serum albumin by spectroscopic methods. Luminescence 26, 710–715 (2011)
Sudlow, G., Birkett, D., Wade, D.: The characterization of two specific drug binding sites on human serum albumin. Mol. Pharmacol. 11, 824–832 (1975)
Sudlow, G., Birkett, D., Wade, D.: Further characterization of specific drug binding sites on human serum albumin. Mol. Pharmacol. 12, 1052–1061 (1976)
Zhao, X., Sheng, F., Zheng, J., Liu, R.: Composition and stability of anthocyanins from purple Solanum tuberosum and their protective influence on Cr(VI) targeted to bovine serum albumin. J. Agric. Food Chem. 59, 7902–7909 (2011)
Sharif-Barfeh, Z., Beigoli, S., Marouzi, S., Rad, A.S., Asoodeh, A., Chamani, J.: Multi-spectroscopic and HPLC studies of the interaction between estradiol and cyclophosphamide with human serum albumin: binary and ternary systems. J. Solut. Chem. 46, 488–504 (2017)
Kamshad, M., Jahanshah Talab, M., Beigoli, S., Sharifirad, A., Chamani, J.: Use of spectroscopic and zeta potential techniques to study the interaction between lysozyme and curcumin in the presence of silver nanoparticles at different sizes. J. Biomol. Struct. Dyn. 37, 2030–2040 (2019)
Zhao, X., Liu, R., Teng, Y., Liu, X.: The interaction between Ag(+) and bovine serum albumin: a spectroscopic investigation. Sci. Total Environ. 409, 892–897 (2011)
Acknowledgements
The authors thank the Medical and Health Technology Development Project of Shandong Province (No. 202013010367) and Yantai Technology and Innovation Development Scheme (No. 2021MSGY048) for financial support.
Author information
Authors and Affiliations
Contributions
JZ: Conceptualization, Methodology, Investigation, Writing—original draft. TW: Conceptualization, Methodology, Investigation, Writing—original draft. SH: Conceptualization, Methodology, Writing—review & editing. JL: Conceptualization, Writing—review & editing. HM: Conceptualization, Methodology, Investigation, Writing—original draft, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, T., Huang, S. et al. Systematic investigations on the biophysical complexation of hydroxyethyl starch 200/0.5 with human serum albumin. J Incl Phenom Macrocycl Chem 102, 743–750 (2022). https://doi.org/10.1007/s10847-022-01155-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-022-01155-0