Abstract
Purpose
A new four-pole connector system (DF-4) for transvenous high-voltage implantable cardioverter defibrillators (ICD) is currently available in clinical practice. However, no clinical data demonstrating the safety and effectiveness of this complex electromechanical design is available. This study aims to test the safety and effectiveness of this newly designed system compared to the conventional DF-1 leads.
Methods
During a 3-year period, 351 consecutive patients were implanted with DF-4 leads as part of an ICD or ICD-cardiac resynchronization therapy system. Patients were matched for age, sex, and follow-up with 154 patients implanted with a standard DF-1 lead. The primary outcome of the study was defibrillation lead failure, defined as the need for lead removal or cap**. Operative, electrical, and safety data were obtained at implant and during postoperative follow-up.
Results
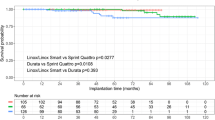
Implantation success rate in both groups was 100 %. A trend towards shorter procedure time was observed in the DF-4 group but the difference did not reach statistical significance. Handling characteristics of the DF-4 leads were graded better than those of DF-1 models. During a total follow-up of 8,130.5 lead-months, there were nine ICD-lead failures (four system erosion/infections and five electrical lead dysfunctions). The overall incidence of electrical lead failure was 0.64 vs. 0.97 per 100 lead-years, for DF-4 and DF-1 leads, respectively (P = 0.2).
Conclusions
This multi-center experience provides strong evidence that the feasibility and safety of this novel technology compare favorably with those of the conventional DF-1 leads.

Similar content being viewed by others
References
Maisel, W. H. (2008). Semper Fidelis: consumer protection for patients with implanted medical devices. The New England Journal of Medicine, 358, 985–987.
Goette, A., Cantu, F., van Erven, L., Geelen, P., Halimi, F., Merino, J. L., et al. (2009). Scientific Initiative Committee of the European Heart Rhythm Association Performance and survival of transvenous defibrillation leads: need for a European data registry. Europace, 11, 31–34.
Maisel, W. H., Moynahan, M., Zuckerman, B. D., Gross, T. P., Tovar, O. H., Tillman, D. B., et al. (2006). Pacemaker and ICD generator malfunction analysis of food and drug administration annual reports. Journal of the American Medical Association, 295, 1901–1906.
Maise, W. H., & Kramer, D. B. (2008). Implantable cardioverter-defibrillator lead performance. Circulation, 117, 2721–2723.
Maisel, W. H., Hauser, R. G., Hammill, S. C., Hauser, R. G., Ellenbogen, K. A., Epstein, A. E., Heart Rhythm Society Task Force on Lead Performance Policies and Guidelines, American College of Cardiology (ACC), American Heart Association (AHA), et al. (2009). Recommendations from the Heart Rhythm Society Task Force on Lead Performance Policies and Guidelines: developed in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm, 6, 869–885.
Ricci, R. P., Pignalberi, C., Magris, B., Aquilani, S., Altamura, V., Morichelli, L., et al. (2012). Can we predict and prevent adverse events related to high-voltage implantable cardioverter defibrillator lead failure? Journal of Interventional Cardiac Electrophysiology, 33(1), 113–121.
Hauser, R. G., & Almquist, A. K. (2008). Learning from our mistakes? Testing new ICD technology. The New England Journal of Medicine, 359, 2517–2519.
Sticherling, C., & Burri, H. (2012). Introduction of new industry standards for cardiac implantable electronic devices: balancing benefits and unexpected risks. Europace, 14, 1081–1086.
Zipes, D. P., Camm, A. J., Borggrefe, M., Buxton, A. E., Chaitman, B., & Fromer, M. (2006). ACC/ AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—executive summary. A report of the American College of Cardiology/American Heart Association task force and the European Society of Cardiology committee for practice guidelines (writing committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death). Circulation, 114, e385–e484.
Forleo, G. B., Della Rocca, D. G., Papavasileiou, L. P., Molfetta, A. D., Santini, L., & Romeo, F. (2011). Left ventricular pacing with a new quadripolar transvenous lead for CRT: early results of a prospective comparison with conventional implant outcomes. Heart Rhythm, 8, 31–37.
Forleo, G. B., Mantica, M., Di Biase, L., Panattoni, G., Della Rocca, D. G., Papavasileiou, L. P., et al. (2012). Clinical and procedural outcome of patients implanted with a quadripolar left ventricular lead: early results of a prospective multicenter study. Heart Rhythm, 9, 1822–1828.
Kleemann, T., Becker, T., Doenges, K., Vater, M., Senges, J., Schneider, S., et al. (2007). Annual rate of transvenous defibrillation lead defects in implantable cardioverter defibrillators over a period of >10 years. Circulation, 115, 2474–2480.
Maisel, W. H. (2007). Transvenous implantable cardioverter-defibrillator leads: the weakest link. Circulation, 115, 2461–2463.
Degeratu, F. T., Khalighi, K., Peters, R. W., Shorofsky, S. R., & Gold, M. R. (2000). Sensing lead failure in implantable defibrillators: a comparison of two commonly used leads. Journal of Cardiovascular Electrophysiology, 11, 21–24.
Borleffs, C. J., van Erven, L., van Bommel, R. J., van der Velde, E. T., van der Wall, E. E., Bax, J. J., et al. (2009). Risk of failure of transvenous implantable cardioverter-defibrillator leads. Circulation: Arrhythmia and Electrophysiology, 2, 411–416.
St. Jude Medical. SJ4 Post Approval Study in Implantable Cardioverter-Defibrillator and Cardiac Resynchronization Therapy Defibrillator Patients. ClinicalTrials.gov Identifier: NCT00940888. http://www.clinicaltrials.gov/ct2/show/NCT00940888. Accessed 24 March 2013
Acknowledgments
The authors thank Cecilia Rubaudo for her assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forleo, G.B., Di Biase, L., Mantica, M. et al. First clinical experience with the new four-pole standard connector for high-voltage ICD leads. Early results of a multicenter comparison with conventional implant outcomes. J Interv Card Electrophysiol 38, 11–18 (2013). https://doi.org/10.1007/s10840-013-9814-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-013-9814-6