Abstract
Purpose
The aim of this study was to compare the addition in culture media of stabilized amorphous calcium carbonate (ACC) versus calcium chloride (CaCl2) or calcium carbonate in crystalline form (CCC) on growth rates among sibling mouse embryos.
Methods
We evaluated the effect of different ACC concentrations on the rates of embryo compaction at 60 h, blastocyst rate at 84 h and percentage of fully hatched at 108 h following hCG injection. As ACC is stabilized by tripolyphosphate (TPP), we also evaluated the addition of TPP alone to the culture media. Finally, we compared supplemented ACC culture media to one-step SAGE and Irvine cleavage media.
Results
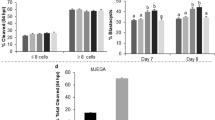
The results revealed that ACC accelerates the compaction and blastocyst rates, as well as the percentage of fully hatched embryos in a dose-dependent manner, with an increased positive effect at 2.5 mM. The magnitude of the effect for ACC-supplemented media on the embryo developmental rate was between 30 to 40% (p < 0.01) faster for each stage, compared to both SAGE and Irvine one-step standard media. Embryos cultured with SAGE or Irvine media with or without supplementation of CaCl2 or CCC, did not produce the same improvements as observed with ACC.
Conclusion
In conclusion, the ACC demonstrates a rapid modulation effect for restoring media optimal pH. ACC can inhibit cathepsin B activity during in vitro culture of fibroblast cells. The beneficial impact of ACC on cleavage mouse embryos is likely due to an improved buffering effect causing slower pH media variations, which may enhance quality and implantation potential of embryos following in vitro culture.











Similar content being viewed by others
References
Shirayoshi Y, Okada TS, Takeichi M. The calcium-dependent cell-cell adhesion system regulates inner cell mass formation and cell surface polarization in early mouse development. Cell. 1983;35(3):631–8.
Zander-Fox DL, Mitchell M, Thompson JG, Lane M. Alterations in mouse embryo intracellular pH by DMO during culture impair implantation and fetal growth. Reprod Biomed Online. 2010;21(2):219–29.
Son HH, Min SH, Yeon JY, et al. Cathepsin B inhibitor, E-64, affects preimplantation development, apoptosis and oxidative stress in pig embryos. Reprod Develop Biol. 2013;37(4):175–83.
Balboula AZ, Yamanaka K, Sakatani M, Hegab AO, Zaabel SM, Takahashi M. Intracellular cathepsin B activity is inversely correlated with the quality and developmental competence of bovine preimplantation embryos. Mol Reprod Dev. 2010;77(12):1031–9.
Balboula AZ, Yamanaka K, Sakatani M, Hegab AO, Zaabel SM, Takahashi M. Cathepsin B activity is related to the quality of bovine cumulus oocyte complexes and its inhibition can improve their developmental competence. Molecular Reproduction and Development: Incorp Gamete Res. 2010;77(5):439–48.
Min SH, Song BS, Yeon JY, et al. A cathepsin B inhibitor, E-64, improves the preimplantation development of bovine somatic cell nuclear transfer embryos. J Reprod Dev. 2014;60(1):21–7.
Riethmacher D, Brinkmann V, Birchmeier C. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc Natl Acad Sci U S A. 1995;92(3):855–9.
Arav A, Aroyo A, Yavin S, Roth Z. Prediction of embryonic developmental competence by time-lapse observation and ‘shortest-half’analysis. Reprod Biomed Online. 2008;17(5):669–75.
Woodward BJ, Montgomery SJ, Hartshorne GM, Campbell KHS, Kennedy R. Spindle position assessment prior to ICSI does not benefit fertilization or early embryo quality. Reprod Biomed Online. 2008;16(2):232–8.
Addadi L, Raz S, Weiner S. Taking advantage of disorder: amorphous calcium carbonate and its roles in biomineralization. Adv Mater. 2003;15(12):959–70.
Meiron OE, Bar-David E, Aflalo ED, et al. Solubility and bioavailability of stabilized amorphous calcium carbonate. J Bone Miner Res. 2011;26(2):364–72.
US Patent US11,052,107.
Shaltiel G, Bar-David E, Meiron OE, et al. Bone loss prevention in ovariectomized rats using stable amorphous calcium carbonate. Health. 2013;5:18–29.
Vaisman N, Shaltiel G, Daniely M, et al. Increased calcium absorption from synthetic stable amorphous calcium carbonate: double-blind randomized crossover clinical trial in postmenopausal women. J Bone Miner Res. 2014;29(10):2203–9.
Hoffman JR, Ben-Zeev T, Zamir A, Levi C, Ostfeld I. Examination of amorphous calcium carbonate on the inflammatory and muscle damage response in experienced resistance trained individuals. Nutrients. 2022;14(9):1894.
A Study Comparing Amorphous Calcium Carbonate (ACC) Versus Crystalline Calcium (CCS) in Hypoparathyroidism Patients (AMCS009). ClinicalTrials.gov Identifier: NCT01815021. A Study Comparing Amorphous Calcium Carbonate (ACC) Versus Crystalline Calcium (CCS) in Hypoparathyroidism Patients. ClinicalTrials.gov Identifier: NCT01815021;. Published online March 20, 2013.https://www.clinicaltrials.gov/study/NCT01815021?term=Amorphical%20&limit=25&page=1&rank=3
Study the Effect of Amorphous Calcium Carbonate (ACC) Treatment on Function and Welfare Improvement in Late-stage Solid Cancer Subjects. ClinicalTrials.gov Identifier: NCT03582280. Study the Effect of Amorphous Calcium Carbonate (ACC) Treatment on Function and Welfare Improvement in Late-stage Solid Cancer Subjects. ClinicalTrials.gov Identifier: NCT03582280; 2018. Published online June 10, 2018.
WO Patent Application 2021181372.
Quinn P, Cooke S. Equivalency of culture media for human in vitro fertilization formulated to have the same pH under an atmosphere containing 5% or 6% carbon dioxide. Fertil Steril. 2004;81(6):1502–6.
Lane M, Ludwig TE, Bavister BD. Phosphate induced developmental arrest of hamster two-cell embryos is associated with disrupted ionic homeostasis. Mol Reprod Dev: Incorp Gamete Res. 1999;54(4):410–7.
Rey C. Calcium phosphates for medical applications. In: Calcium phosphates in biological and industrial systems. Springer Published online; 1998. p. 217–51.
Kane MT. The effects of pH on culture of one-cell rabbit ova to blastocysts in bicarbonate-buffered medium. Reproduction. 1974;38(2):477–80.
Dale B, Menezo Y, Cohen J, DiMatteo L, Wilding M. Intracellular pH regulation in the human oocyte. Hum Reprod. 1998;13(4):964–70.
Chang B, Liao M, Kuo MY, Chen C. Developmental toxicity of arecoline, the major alkaloid in betel nuts, in zebrafish embryos. Birth Defects Res A Clin Mol Teratol. 2004;70(1):28–36.
Morbeck DE, Krisher RL, Herrick JR, Baumann NA, Matern D, Moyer T. Composition of commercial media used for human embryo culture. Fertil Steril. 2014;102(3):759–766.e9.
Brinster RL. Studies on the development of mouse embryos in vitro. I. The effect of osmolarity and hydrogen ion concentration. J Experiment Zoology. 1965;158(1):49–57.
Ferguson WJ, Braunschweiger KI, Braunschweiger WR, et al. Hydrogen ion buffers for biological research. Anal Biochem. 1980;104(2):300–10.
Edwards LJ, Williams DA, Gardner DK. Intracellular pH of the preimplantation mouse embryo: effects of extracellular pH and weak acids. Mol Reprod Dev: Incorp Gamete Res. 1998;50(4):434–42.
Barr KJ, Garrill A, Jones DH, Orlowski J, Kidder GM. Contributions of Na+/H+ exchanger isoforms to preimplantation development of the mouse. Mol Reprod Dev: Incorp Gamete Res. 1998;50(2):146–53.
Behr BR, Stratton CJ, Foote WD, Knutzen V, Sher G. In vitro fertilization (IVF) of mouse ova in HEPES-buffered culture media. J in vitro fertil embryo transf. 1990;7(1):9–15.
Bhattacharyya A, Yanagimachi R. Synthetic organic pH buffers can support fertilization of guinea pig eggs, but not as efficiently as bicarbonate buffer. Gamete Res. 1988;19(2):123–9.
El-Danasouri I, Selman H, Strehler E, de Santo M, Sterzik K. Comparison of MOPS and HEPES buffers during vitrification of human embryos. Hum Reprod. 2004;14:i136.
Barrett AJ, Kirschke H. [41] Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol. 1981;80:535–61.
Tsukamoto S, Hara T, Yamamoto A, et al. Functional analysis of lysosomes during mouse preimplantation embryo development. J Reprod Dev. 2013;59(1):33–9.
Funding
This research was funded by Amorphical Ltd.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.A. and P.P.; methodology and performing experiments, A.A.; and Y.N. and Y.P.; formal analysis, AA and Y.N. and T.H.; writing—original draft preparation, A.A. and Y.D.B; writing—review and editing, A.A. and Y.N. and T.H. and Y.P. and Y.D.B. and P.P. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Y.N, T.H, Y.P, and Y.D.B are employees of Amorphical Ltd.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arav, A., Natan, Y., Hejja, T. et al. The effect of amorphous calcium carbonate as a culture media supplement on embryonic development of murine sibling embryos. J Assist Reprod Genet 40, 2409–2418 (2023). https://doi.org/10.1007/s10815-023-02899-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02899-5