Abstract
The chrysophyte Poterioochromonas malhamensis has potential for controlling algal blooms through rapid grazing of toxic Microcystis cells and efficient degradation of microcystin. However, this method has not been used in practice because a high-cell-density method for cultivating P. malhamensis has not yet been established and the actual effect of the chrysophyte in controlling Microcystis blooms in the field is still unknown. To achieve the application of this method, high-cell-density heterotrophic cultivation of P. malhamensis was established through optimizing the carbon/glucose concentration, C:N ratio, temperature, pH, and dissolved oxygen concentration. Under optimized conditions, the cell concentration of P. malhamensis reached more than 3 × 108 cells mL−1, which exceeds that reported in other studies by more than an order of magnitude. The ability of the chemoheterotrophic P. malhamensis to graze unicellular Microcystis cells was comparable to that of autotrophic and phagotrophic P. malhamensis. A controlled field experiment showed that chemoheterotrophic P. malhamensis could live in the aquatic environment with a Microcystis bloom and decrease the Microcystis biomass on the surface of the water by promoting the sedimentation of colonial Microcystis cells. This study offers an opportunity to drive the development of methods to control Microcystis blooms using predatory P. malhamensis.


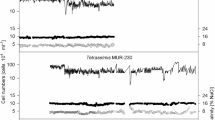
source at this point is running out and the glucose concentration is too low to be detected. Error bars represent the standard deviation (n = 3)





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahn CY, Park MH, Joung SH, Kim HS, Jang KY, Oh HM (2003) Growth inhibition of cyanobacteria by ultrasonic radiation: laboratory and enclosure studies. Environ Sci Technol 37:3031–3037
Andersen RA, Graf L, Malakhov Y, Yoon HS (2017) Rediscovery of the Ochromonas type species Ochromonas triangulata (Chrysophyceae) from its type locality (Lake Veysove, Donetsk region, Ukraine). Phycologia 56:591–604
Blom JF, Pernthaler J (2010) Antibiotic effects of three strains of chrysophytes (Ochromonas, Poterioochromonas) on freshwater bacterial isolates. FEMS Microbiol Ecol 71:281–290
Boenigk J, Stadler P (2004) Potential toxicity of chrysophytes affiliated with Poterioochromonas and related ‘Spumella-like’flagellates. J Plankton Res 26:1507–1514
Boon PI, Bunn SE, Green JD, Shiel RJ (1994) Consumption of cyanobacteria by freshwater zooplankton: implications for the success of ‘top-down’ control of cyanobacterial blooms in Australia. Mar Freshwater Res 45:875–887
Boxhorn JE, Holen DA, Boraas ME (1998) Toxicity of the chrysophyte flagellate Poterioochromonas malhamensis to the rotifer Brachionus angularis. Hydrobiologia 387:283–287
Calbet A, Landry MR (2004) Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems. Limnol Oceanogr 49:51–57
Chen JL, Proteau PJ, Roberts MA, Gerwick WH, Slate DL, Lee RH (1994) Structure of malhamensilipin A, an inhibitor of protein tyrosine kinase, from the cultured chrysophyte Poterioochromonas malhamensis. J Nat Prod 57:524–527
Corno G, Jurgens K (2006) Direct and indirect effects of protist predation on population size structure of a bacterial strain with high phenotypic plasticity. Appl Environ Microbiol 72:78–86
Destain J, Haubruge É, Thonart P, Portetelle D, Francis F, Bauwens J, Tarayre C, Brasseur C, Mattéotti C, Vandenbol M (2014) Isolation of an amylolytic chrysophyte, Poterioochromonas sp., from the digestive tract of the termite Reticulitermes santonensis. Biotechnol Agron Soc Environ 18:19–31
Fenchel T (2014) Protozoa and oxygen. Acta Protozool 53:3–12
Ger KA, Naus-Wiezer S, De Meester L, Lürling M (2019) Zooplankton grazing selectivity regulates herbivory and dominance of toxic phytoplankton over multiple prey generations. Limnol Oceanogr 64:1214–1227
Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu Rev Plant Biol 56:99–131
He X, Wert EC (2016) Colonial cell disaggregation and intracellular microcystin release following chlorination of naturally occurring Microcystis. Water Res 101:10–16
He Y, Ma M, Hu Q, Gong Y (2021) Assessment of NH4HCO3 for the control of the predator flagellate Poterioochromonas malhamensis in pilot-scale culture of Chlorella sorokiniana. Algal Res 60:102481
Holen DA, Boraas ME (1995) Mixotrophy in chrysophytes. In: Sandgren CD, Smol JP, Kristiansen J (eds) Chrysophyte algae: ecology, phylogeny and development. Cambridge University Press, Cambridge, pp 119–140
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87:38–46
Humphries S, Widjaja F (1979) A simple method for separating cells of Microcystis aeruginosa for counting. Br Phycol J 14:313–316
** H, Zhang H, Zhou Z, Li K, Hou G, Xu Q, Chuai W, Zhang C, Han D, Hu Q (2020) Ultrahigh-cell-density heterotrophic cultivation of the unicellular green microalga Scenedesmus acuminatus and application of the cells to photoautotrophic culture enhance biomass and lipid production. Biotechnol Bioeng 117:96–108
Kauss H, Kriebitzsch C (1969) Demonstration and partial purification of A β-(1→3)-glucan phosphorylase. Biochem Biophys Res Comm 35:926–930
Kim BR, Han MS (2007) Growth and grazing of the mixotrophic flagellate Poterioochromonas malhamensis on the cyanobacterium Microcystis aeruginosa. Kor J Nat Conserv 5:183–194
Leakey R, Burkill P, Sleigh M (1994) A comparison of fixatives for the estimation of abundance and biovolume of marine planktonic ciliate populations. J Plankton Res 16:375–389
Lewitus AJ, Caron DA (1991) Physiological responses of phytoflagellates to dissolved organic substrate additions. 1. Dominant role of heterotrophic nutrition in Poterioochromonas malhamensis (Chrysophyceae). Plant Cell Physiol 32:671–680
Li M, **ao M, Zhang P, Hamilton DP (2018) Morphospecies-dependent disaggregation of colonies of the cyanobacterium Microcystis under high turbulent mixing. Water Res 141:340–348
Lin Z, Raya A, Ju LK (2014) Microalga Ochromonas danica fermentation and lipid production from waste organics such as ketchup. Process Biochem 49:1383–1392
Liu J, Sun Z, Chen F (2014) Heterotrophic production of algal oils. In: Pandey A, Lee D-J, Chisti Y, Soccol CR (eds) Biofuels from algae. Elsevier, Amsterdam, pp 111–142
Liu M, Shi X, Chen C, Yu L, Sun C (2017) Responses of Microcystis colonies of different sizes to hydrogen peroxide stress. Toxins 9:306
Lyu K, Gu L, Wang H, Zhu X, Zhang L, Sun Y, Huang Y, Yang Z (2019) Transcriptomic analysis dissects the mechanistic insight into the Daphnia clonal variation in tolerance to toxic Microcystis. Limnol Oceanogr 64:272–283
Ma M, Gong Y, Hu Q (2018) Identification and feeding characteristics of the mixotrophic flagellate Poterioochromonas malhamensis, a microalgal predator isolated from outdoor massive Chlorella culture. Algal Res 29:142–153
Ma M, Yuan D, He Y, Park M, Gong Y, Hu Q (2017) Effective control of Poterioochromonas malhamensis in pilot-scale culture of Chlorella sorokiniana GT-1 by maintaining CO2- mediated low culture pH. Algal Res 26:436–444
Nguyen TDP, Tran TNT, Le TVA, Phan TXN, Show PL, Chia SR (2019) Auto-flocculation through cultivation of Chlorella vulgaris in seafood wastewater discharge: influence of culture conditions on microalgae growth and nutrient removal. J Biosci Bioeng 127:492–498
Ou D, Song L, Gan N, Chen W (2005) Effects of microcystins on and toxin degradation by Poterioochromonas sp. Environ Toxicol 20:373–380
Rigosi A, Carey CC, Ibelings BW, Brookes JD (2014) The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol Oceanogr 59:99–114
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111:1–61
Roderer G (1986) Poterioochromonas malhamensis-a unicellular alga as test system in ccotoxicology, toxicology, and pharmacology. Environ Toxicol 1:123–138
Rottberger J, Gruber A, Boenigk J, Kroth PG (2013) Influence of nutrients and light on autotrophic, mixotrophic and heterotrophic freshwater chrysophytes. Aquat Microb Ecol 71:179–191
Sanders RW, Porter KG, Caron DA (1990) Relationship between phototrophy and phagotrophy in the mixotrophic chrysophyte Poterioochromonas malhamensis. Microb Ecol 19:97–109
Sarnelle O (1992) Nutrient enrichment and grazer effects on phytoplankton in lakes. Ecology 73:551–560
Shams S, Cerasino L, Salmaso N, Dietrich DR (2014) Experimental models of microcystin accumulation in Daphnia magna grazing on Planktothrix rubescens: implications for water management. Aquat Toxicol 148:9–15
Sherr EB, Sherr BF (2002) Significance of predation by protists in aquatic microbial food webs. Antonie Van Leeuwenhoek 81:293–308
Tan CK, Johns MR (1991) Fatty acid production by heterotrophic Chlorella saccharophila. Hydrobiologia 215:13–19
Tan X, Duan Z, Duan P, Parajuli K, Newman J, Shu X, Zhang D, Gao L, Li M (2020) Flocculation of Microcystis unicells induced by pH regulation: mechanism and potential application. Chemosphere 263:127708
Toda N, Murakami H, Kanbara A, Kuroda A, Hirota R (2021) Phosphite reduces the predation impact of Poterioochromonas malhamensis on cyanobacterial culture. Plants 10:1361
Touloupakis E, Cicchi B, Benavides AM, Torzillo G (2016) Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.). Appl Microbiol Biotechnol 100:1333–1341
Van Wichelen J, Vanormelingen P, Codd GA, Vyverman W (2016) The common bloom-forming cyanobacterium Microcystis is prone to a wide array of microbial antagonists. Harmful Algae 55:97–111
Wang H, Tao Y, Li Y, Wu S, Li D, Liu X, Han Y, Manickam S, Show PL (2021a) Application of ultrasonication at different microbial growth stages during apple juice fermentation by Lactobacillus plantarum: investigation on the metabolic response. Ultrason Sonochem 73:105486
Wang W, Zhang Y, Shen H, **e P, Yu J (2015) Changes in the bacterial community and extracellular compounds associated with the disaggregation of Microcystis colonies. Biochem Syst Ecol 61:62–66
Wang X, Li H, Zhan X, Ma M, Yuan D, Hu Q, Gong Y (2021b) Development and application of quantitative real-time PCR based on the mitochondrial cytochrome oxidase subunit I gene for early detection of the grazer Poterioochromonas malhamensis contaminating Chlorella culture. Algal Res 53:102133
Wang Y, Gong Y, Dai L, Sommerfeld M, Zhang C, Hu Q (2018) Identification of harmful protozoa in outdoor cultivation of Chlorella and the use of ultrasonication to control contamination. Algal Res 31:298–310
Wen Z, Chen F (2000) Heterotrophic production of eicosapentaenoid acid by the diatom Nitzschia laevis: effects of silicate and glucose. J Ind Microbiol Biotechnol 25:218–224
**ao M, Li M, Reynolds CS (2018) Colony formation in the cyanobacterium Microcystis. Biol Rev 93:1399–1420
Zeković DB, Kwiatkowski S, Vrvić MM, Jakovljević D, Moran CA (2005) Natural and modified (1→ 3)-β-D-glucans in health promotion and disease alleviation. Crit Rev Biotechnol 25:205–230
Zhang L, Gu L, Hou X, Kong Q, Chen K, Zhu X, Huang Y, Chen Y, Yang Z (2018) Chlorophytes prolong mixotrophic Ochromonas eliminating Microcystis: temperature-dependent effect. Sci Total Environ 639:705–713
Zhang L, Wang Z, Wang N, Gu L, Sun Y, Huang Y, Chen Y, Yang Z (2020) Mixotrophic Ochromonas addition improves the harmful Microcystis-dominated phytoplankton community in in situ microcosms. Environ Sci Technol 54:4609–4620
Zhang X, Hu HY, Men YJ, Yang J, Christoffersen K (2009) Feeding characteristics of a golden alga (Poterioochromonas sp.) grazing on toxic cyanobacterium Microcystis aeruginosa. Water Res 43:2953–2960
Zhang X, Hu HY, Hong Y, Yang J (2008) Isolation of a Poterioochromonas capable of feeding on Microcystis aeruginosa and degrading microcystin-LR. FEMS Microbiol Lett 288:241–246
Zhang X, Hu HY, Warming TP, Christoffersen KS (2011) Life history response of Daphnia magna to a mixotrophic golden alga, Poterioochromonas sp., at different food levels. Bull Environ Contam Toxicol 87:117–123
Zhang X, Watanabe MM (2001) Grazing and growth of the mixotrophic chrysomonad Poterioochromonas malhamensis feeding on algae. J Phycol 37:738–743
Zhang X, Watanabe MM (1996) Light and electron microscopy of grazing by Poterioochromonas malhamensis (Chrysophyceae) on a range of phytoplankton taxa. J Phycol 32:37–46
Zheng Y, Li T, Yu X, Bates PD, Dong T, Chen S (2013) High-density fed-batch culture of a thermotolerant microalga Chlorella sorokiniana for biofuel production. Appl Energy 108:281–287
Acknowledgements
Thanks are due to Dr. Binliang Wang and Qingyang Song for hel** with the outdoor control experiment. The authors also thank the National Aquatic Biological Resource Center (NABRC) at the Institute of Hydrobiology, Chinese Academy of Sciences, for providing support.
Funding
This work was funded by the National Key Research and Development Program of China (No. 2019YFD0900302), the National Natural Science Foundation of China (No. 31772419, No. 31872201, and No. 32002413), the National Key Research and Development Project (No. 2017YFE0125700), the China Postdoctoral Science Foundation (No. 2019M662749), and the Agricultural Science and Technology Innovation Action Project of Hubei Province of China (2018).
Author information
Authors and Affiliations
Contributions
Mingyang Ma and Fuchen Wang performed the experiments, analyzed the data, and wrote the paper. Chaojun Wei and Hongxia Wang participated in the outdoor experiment. Jian** Chen and Hu ** participated in the fermentation of P. malhamensis in the 7.5-L bioreactors. Lirong Song provided algal cultures and offered crucial suggestions on the analysis of the results. Qiang Hu and Yingchun Gong contributed to the design of the experiments, the drafting of the paper, and revising it critically. All authors gave approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, M., Wang, F., Wei, C. et al. Establishment of high-cell-density heterotrophic cultivation of Poterioochromonas malhamensis contributes to achieving biological control of Microcystis. J Appl Phycol 34, 423–434 (2022). https://doi.org/10.1007/s10811-021-02659-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02659-x