Abstract
We aimed to identify unique constellations of sensory phenotypes for genetic etiologies associated with diagnoses of autism spectrum disorder (ASD) and intellectual disability (ID). Caregivers reported on sensory behaviors via the Sensory Profile for 290 participants (younger than 25 years of age) with ASD and/or ID diagnoses, of which ~ 70% have a known pathogenic genetic etiology. Caregivers endorsed poor registration (i.e., high sensory threshold, passive behaviors) for all genetic subgroups relative to an “idiopathic" comparison group with an ASD diagnosis and without a known genetic etiology. Genetic profiles indicated prominent sensory seeking in ADNP, CHD8, and DYRK1A, prominent sensory sensitivities in SCN2A, and fewer sensation avoidance behaviors in GRIN2B (relative to the idiopathic ASD comparison group).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Neurodevelopmental disorders (APA, 2013) such as autism spectrum disorder (ASDFootnote 1) and intellectual disability (ID) are associated with a range of atypical or unique sensory behaviors (Ben-Sasson et al., 2009; Dellapiazza et al., 2021; Joosten & Bundy, 2010) that may serve as early emerging prognostic markers in prediction of later skills (Baranek et al., 2017; Williams et al., 2018). For instance, as early as 9–18 months of age, infants who are later diagnosed with ASD exhibit differences in auditory processing, avoidant or inconsistent use of eye contact, over-responsivity to tactile input, and attention and arousal impairments (Tomchek & Dunn, 2007; Tomchek et al., 2014). Upwards of 45–95% of young individuals with ASD and/or ID diagnoses are reported to exhibit behaviors associated with sensory differences, including sensitivity (e.g., covering ears to loud or unexpected sounds; restricted food preferences), poor sensory registration (e.g., failure to orient towards sounds or react to pain), or sensory seeking (e.g., rocking, hand flap**, noise-making) (Bizzell et al., 2020; Grzadzinski et al., 2020; Lane et al., 2010; Little et al., 2015). Other young individuals avoid sensory stimuli such as particular sounds, textures of play materials or clothing, and foods (Smith et al., 2005; Talay-Ongan & Wood, 2000), which may restrict learning opportunities that result from active exploration of the environment (Baranek et al., 2002). In addition, there are extreme consequences to sensory sensitivities, such as medical problems due to restricted diet, severe aggression or self-injurious behavior in response to sensory stimuli, or difficulty managing/accessing public spaces (Kurtz-Nelson et al., 2021; Talay-Ongan & Wood, 2000).
Despite the prevalence of sensory behaviors and sensitivities in ASD samples, there is limited information about whether potential subgroups exhibit unique constellations of sensory behaviors that could be considered a phenotypic profile. One possible avenue to uncovering biologically derived subgroups implements what is known as a genetics-first approach (Stessman et al., 2014), in which research targets individuals with an identified genetic etiology to complete in-depth phenoty** across clinical, medical, behavioral, and neural levels. Approximately 25% of autistic individuals have an identified genetic etiology presumed to underlie their ASD and/or ID diagnoses, typically in the form of likely pathogenic genetic disruptions, such as de novo copy number variants, missense mutations, and protein-truncating likely gene-disrupting (LGD) mutations (Iossifov et al., 2012, 2014; Kaufman et al., 2010; McCarthy et al., 2014). To date, although many case studies targeting human phenotypes associated with specific genes report sensory atypicalities, few efforts have systematically addressed sensory behaviors pertaining to high-confidence genetic etiologies.
We focus our efforts on building a deeper sensory phenotype for five genetic subgroups: ADNP, CHD8, DYRK1A, GRIN2B, and SCN2A. These genes were selected due to empirical evidence of relevant sensory behaviors (described in the next section), as well as feasible access to sufficient data for analysis. Given limited information from the extant literature, we describe work from humans and animals that highlights primary sensory behaviors, as well as secondary factors that may implicate neurobehaviors that may underlie sensory processing.
Genetic Subgroups and Relevant Sensory Behaviors
ADNP encodes for activity dependent neuroprotective protein that serves as a transcription factor-encoding gene for chromatin remodeling implicated in ASD (~ 0.2% of cases) and perhaps more notably within ID (Arnett et al., 2018; Gozes et al., 2017; Helsmoortel et al., 2014). Recent work highlights a dominant sensory reactivity phenotype for ADNP with high levels of sensory seeking behaviors across multiple sensory domains (e.g., tactile, auditory, visual) assessed via parental report and clinician-administered observation (Siper et al., 2021).
CHD8 encodes for chromodomain helicase DNA binding protein 8 that regulates genetic expression through chromatin remodeling and affects prenatal neuronal development (Thompson et al., 2008). CHD8 is among the most prevalent of genes enriched for LGD mutations among large ASD samples (Barnard et al., 2015), for instance, accounting for 9 out of 2573 cases (0.35%) of the Simons Simplex Collection (Fischbach & Lord, 2010). Deep-phenoty** efforts of CHD8 indicate high rates of ASD diagnoses, moderate rates of ID diagnoses, common facial features, gastrointestinal issues, sleep issues, and macrocephaly (Bernier et al., 2017). Our sample included children, adolescents, and young adults with a wide range of motor, cognitive, and language phenotypes. Thus, in analysis 1, we assessed variation in sensory behaviors in the context of individual differences relevant to genetic subgroup. First, we examined the impact of diagnostic status (e.g., diagnosis of ASD and/or ID), genetic etiology (e.g., presence of a known and likely pathogenic disruptive mutation or copy number variation), biological sex assigned at birth, and functional motor and language abilities (currently walking and talking) on sensory behaviors. We hypothesized that given prior evidence described above, both ASD and ID diagnoses would impact sensory behaviors. Both ASD samples and genetic subgroups experience motor delays such as late walking relative to non-ASD samples (Lavenne-Collot et al., 2021; Lee et al., 2020) and language delays are prominent in ASD (Delehanty et al., 2018; Wodka et al., 2013), reported in participants with disruptive mutations in GRIN2B (Mishra et al., 2016; Platzer et al., 2017), and observed in other genetic subgroups (Bernier et al., Analytic Plan All analyses were performed using R (version 4.0.3). First (analysis 1), in the full sample (saturated with ID diagnoses and known genetic etiologies compared to other samples in the extant literature), we used single-level regressions to test what factors were predictive of Sensory Profile sensory quadrants. Four models were run corresponding to the four quadrants: registration, sensation seeking, sensitivity, and avoidance. In addition, each model included the following independent variables as fixed and non-interacting effects: ASD diagnosis, ID diagnosis, genetic etiology (known versus no known etiology), currently walking, currently talking, and sex. All fixed effects were inputted at the same time to better understand the relative effect of each predictor. The fixed intercept represents a female participant without an ASD or ID diagnosis, without a known genetic etiology, and who is not walking or talking. In this way, F-tests indicate the assessment of all regression coefficients simultaneously and estimated coefficients were then computed as the fixed effects estimates via t-tests to examine each regression coefficient (i.e., one at a time). Models were reviewed to ensure that variance inflation factors (VIF) were less than 5 to ensure a lack of multicollinearity (Craney & Surles, 2002) and all predictors exhibited a VIF < 1.49. Second (analysis 2), we examined subgroup differences by directly testing the idiopathic ASD comparison group to participants with one of five genetic events: ADNP, CHD8, DYRK1A, GRIN2B, or SCN2A. In this analysis, a single fixed effect group variable was entered into a linear mixed effect model for each quadrant score. Post-hoc correction of multiple comparisons accounted for Benjamini-Hochberg (FDR) correction to adjust p-values given the multiplicity of testing Sensory Profile outcomes. Third (exploratory analysis), we utilized classification and regression tree analysis (CART) using the rpart package (Therneau & Atkinson, 2019) per (Laton et al., 2014) to identify unique sensory feature profiles for each target genetic subgroup relative to the idiopathic ASD comparison group. In this way, all sensory measurements were input into an unsupervised model that computed thresholds that maximally distinguished idiopathic ASD (selected as 0) and genetic subgroup (selected as 1). CART is a non-parametric approach that differentiates groups of observations (i.e., participants) using recursive modeling of categorical and/or continuous predictors. At each step of the analysis, CART splits the sample according to thresholds on predictor variables, called nodes. At each node, a predictor is selected that maximally distinguishes the groups. This is repeated until the sample is fully divided, or when it reaches a predetermined criterion (current analysis: minimum split = 15, minimum final bucket = 5) for the number of classified participants at a terminal node. Predictor variables included the four quadrant scores. Multivariate classification (tenfold, 100 iterations) was conducted using the “convenience layer for classification in R” toolbox (Laton, 2020) to determine how well the model predicted classification of ASD versus genetic subgroup.
Results
Sensory Behaviors Based Upon Individual Difference Factors (Analysis 1)
Here, we describe which individual difference factors indicated significance across the full sample (n = 290), including the idiopathic ASD comparison group (n = 87), targeted genetic subgroups (21 ADNP, 21 CHD8, 24 DYRK1A, 34 GRIN2B, 39 SCN2A), and other known genetic etiologies (n = 64).
Table 2 reports omnibus F-test values (i.e., all effects as assessed simultaneously). Figure 1 illustrates the effect of each single regression with the estimated coefficient and 95% confidence interval reported and plotted and t-test significance signified by the marker style. Intercept values indicate the estimated marginal mean for each quadrant score. Independent variable values indicate the extent by which that factor impacts the intercept value, such that negative coefficients indicate lower quadrant scores (i.e., greater likelihood that the participant is experiencing difficulties in the quadrant). None of the models indicated that sensory quadrants varied by sex, p > 0.45, and only one trend was observed for those currently walking (decreased sensory seeking behaviors, p = 0.086).
Effects of individual difference predictors on Sensory Profile quadrants in the full sample (N = 290). Results of regression models of the independent variables of autism spectrum disorder (ASD) diagnosis, intellectual disability (ID) diagnosis, currently talking, currently walking, known genetic etiology, or sex for each sensory feature. The effect of a predictor variable is marked by a central marker with its 95% confidence interval presented along a horizontal line. Triangular red markers indicate a significant effect (p < .05), square purple markers indicate a trend (p < .1), and circular black markers indicate a nonsignificant effect (p > .1). The Intercept indicates the mean value for each quadrant. Fixed effect values indicate the change from the intercept. Directionality was designated as the effect of having the diagnosis (ASD, ID), currently exhibiting the skill (Talking, Walking), having a known genetic etiology (Gene), or the effect of being female (Sex). In this way, positive standardized coefficients indicate decreased endorsement of sensory behaviors, and negative standardized coefficients indicate increased endorsement of sensory behaviors
Registration (Q1) had the lowest overall estimated marginal mean (2.87) and omnibus tests indicated that all predictors were significant when assessed simultaneously except for sex. Patterns suggested negative coefficient scores reflective of poor registration behaviors for participants with an ID diagnosis (non-significant as single regression, p = 0.58) and/or a known genetic etiology (p < 0.001). Positive coefficient scores indicated fewer poor registration behaviors for participants with an ASD diagnosis (p = 0.33), those who are currently walking (p = 0.18), and those who are currently talking (p = 0.003). Sensation seeking (Q2) had a moderately high estimated marginal mean (4.02). Negative coefficient scores indicated more sensation seeking behaviors for participants with an ID diagnosis (p = 0.040) and those who were currently talking (p = 0.005). Sensory sensitivities (Q3) had a moderately high estimated marginal mean (3.88). Negative coefficient scores indicated more sensory sensitivities for participants who were currently talking (p = 0.044). Sensory avoidance (Q4) had a moderately high estimated marginal mean (3.65). Positive coefficient scores indicated that participants with an ID diagnosis experienced fewer avoidance behaviors (p = 0.037).
To summarize across individual difference factors: Individuals with an ASD diagnosis had decreased registration behaviors. Individuals with an ID diagnosis had poor registration and seeking behaviors, but decreased avoidance behaviors. Individuals that are currently talking (i.e., at minimum, phrase speech) had increased seeking and sensitivity behaviors, but fewer poor registration behaviors. Individuals that are currently walking had fewer poor registration behaviors. Individuals with a known genetic etiology had poor registration behaviors. No significant differences were found based upon sex.
Sensory Behaviors for Specific Genetic Subgroups (Analysis 2)
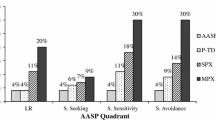
Second, sensory phenotypes were explored for genetic subgroups of ADNP, CHD8, DYRK1A, GRIN2B, and SCN2A, relative to each other and the idiopathic ASD comparison group. Comparisons were not conducted against the “other” known genetic etiology group given the wide range of variability in function of the genes (n = 64 across 29 different genes). Quadrant scores for each group are illustrated in Fig. 2 and reported in Table 1. From the four statistical models, overall group differences were noted for registration, F(1, 220) = 7.51, p < 0.0001, seeking, F(1, 220) = 4.11, p = 0.001, and avoidance, F(1, 220) = 3.23, p = 0.008, but not sensitivity, F(1, 220) = 1.27, p = 0.28. Here, we focus on each genetic subgroup relative to the idiopathic ASD comparison group (all p-values adjusted using FDR). The ADNP group exhibited poor registration (p = 0.005) and seeking (p = 0.026) behaviors. The CHD8 group exhibited poor registration (p = 0.006) behaviors. The DYRK1A group exhibited poor registration (p = 0.029) and seeking (p = 0.032) behaviors. The GRIN2B group exhibited poor registration (p = 0.0002) and seeking (p = 0.29) behaviors but decreased avoidance (p = 0.021) behaviors. The SCN2A group exhibited poor registration (p < 0.0001) behaviors.
Sensory Profile quadrant scores for genetic subgroups and the comparison “idiopathic” ASD group. Violin plot highlights relative density and boxplots illustrate quartile values. Lower Sensory Profile scores indicate greater likelihood of sensory behaviors (1 = Always; 2 = Frequent, 3 = Occasional, 4 = Seldom, 5 = Never)
Classification of Sensory Behaviors for Genetic Subgroups Relative to Idiopathic ASD (Exploratory Analysis)
Lastly, this exploratory analysis identified the sensory behaviors that were most likely to separate each genetic subgroup relative to the idiopathic ASD comparison group with the goal of using the sensory behaviors to accurately classify each genetic subgroup. Results from the cross-validation procedure are presented in Table 3. Generally, models exhibited: (1) modest accuracy (all > 71.1% accurate for classifying the genetic subgroup); (2) variable sensitivity indicating many false negatives for SCN2A, 47%, such that many SCN2A were incorrectly classified as the idiopathic ASD comparison group, and (3) variable specificity (e.g., many false positives for ADNP, CHD8 and DYRK1A, such that many of the idiopathic ASD comparison group were incorrectly classified with the genetic subgroup). All classification trees are illustrated in Fig. 3, and results are described by the sensory feature that designates branch “splits”. Below we describe the classification bins (final box, known as ‘leaves’) as either being an idiopathic or subgroup leaf (top line of box) based upon the proportion of subjects in that leaf (n idiopathic ASD on left, n genetic subgroup on right) and the proportion of the sample (87 idiopathic ASD and n for full subgroup) in that leaf. Broadly speaking, across most models, trees were first split by registration scores with a large idiopathic ASD leaf classified by decreased registration issues.
Classification and regression tree results for each genetic subgroup. All quadrant scores were input in the classification and regression tree analysis, and subsequent results demonstrated the scores that best differentiated idiopathic autism spectrum disorder (ASD) from each genetic subgroup. Square boxes indicate the majority group in each leaf (top text), the number of ASD participants in each leaf (left middle), the number of genetic subgroup participants in each leaf (right middle), and the proportion of the overall sample (bottom percentage, relative to combined participants from both groups). Tree branches are indicated by dashed lines and the variable that splits each branch is noted in bold with the score separating the left and right branch. Depth of color illustrates the likelihood of belonging to the idiopathic ASD group (grey shading) or the genetic subgroup (ADNP, orange; CHD8, red; DYRK1A, purple; GRIN2B, green; SCN2A, blue)
Although 42.9% of the ADNP group (9 out of 21) were classified in an idiopathic ASD leaf, two ADNP classification leaves were found: First, 38% of the ADNP group were classified by having poor registration (Q1 < 3.2) and seeking (Q2 < 3.4) behaviors but decreased avoidance (Q4 ≥ 2.7) behaviors. Second, 19% of the ADNP group were classified by increased sensory seeking (Q2 < 3.4) behaviors but decreased registration (Q1 ≥ 3.2) or avoidance (Q4 ≥ 3.7) behaviors.
A majority 57.1% of the CHD8 group (12 out of 21) were classified in an idiopathic ASD leaf. However, two CHD8 classification leaves were found, both classified by an initial tree branch based upon poor registration (Q3 < 3.1): The first classified 23.8% of the CHD8 group by decreased sensitivity (Q3 ≥ 3.8) behaviors. The second classified 19.0% of the CHD8 group with increased sensitivity (Q3 < 3.8) behaviors but decreased registration (i.e., Q1 scores between 2.7 and 3.1) behaviors.
Only 32% of the DYRK1A group (8 out of 25) were classified in an idiopathic ASD leaf. Three DYRK1A classification leaves were found, all indicating increased sensory seeking (Q2 < 4.4) behaviors. First, 28% of the DYRK1A group were classified by decreased avoidance (Q4 ≥ 4.1) behaviors. The second and third DYRK1A groups were classified by avoidance behaviors (Q4 < 4.1) and “frequent” registration (Q1 between 2.2 and 3.2) behaviors. These groups were differentiated by sensory seeking with the second group (24% of the DYRK1A group) classified increased seeking (Q2 < 2.9) behaviors and the third group (12% of the DYRK1A group) classified by decreased seeking (Q2 ≥ 3.9) behaviors.
Only 27.2% of the GRIN2B group (9 out of 33) were classified in an idiopathic ASD leaf. Two GRIN2B classification leaves were found, both indicating decreased avoidance (Q4’s > 3.2) behaviors: First, 54.5% of the GRIN2B group were classified by poor registration (Q1 < 3.2) behaviors. Second, 21.2% of the GRIN2B group were classified by “occasional” registration behaviors (Q1 scores between 3.2 and 3.9).
Lastly, 28.2% of the SCN2A group (11 out of 39) were classified in an idiopathic ASD leaf. Two SCN2A classification leaves were found, all indicating poor registration (Q1 < 3.4). First, 53.8% of the SCN2A group were classified by decreased sensitivity (Q3 ≥ 3.6) behaviors. Second, 17.9% of the SCN2A group were classified by increased sensitivity (Q3 < 3.6) and registration (Q1 < 2.5) behaviors but decreased seeking (Q2 ≥ 3.2) behaviors.
Discussion
Here, we sought to characterize the extent to which sensory behaviors vary based upon diagnosis (ASD and/or ID), presence of a known genetic etiology, sex, and functional abilities (currently walking and/or talking). As our primary measure, we used the caregiver report from a common sensory measure: the 125-item Sensory Profile (Dunn, 1999). The current sample of this study is saturated for genetic etiologies and co-occurring ID with the goal to examine group-level differences or relevant individual difference factors and identify possible sensory phenotypes related to specific genetic subgroups, including ADNP, CHD8, DYRK1A, GRIN2B, and SCN2A.
Addressing Individual Difference Factors Relevant to Genetic Subgroups
Because we sought to address how individual difference factors may uniquely yet collectively impact sensory behaviors, we examined the effects of each factor when considered in concert with other phenotypic aspects. This approach has been successful to delineate complex behaviors in genomics (Hudac et al., 2020; Lippert et al., 2013; Yang et al., 2014), as well as within classic psychological approaches in ASD research (Kaat et al., 2021; Tillmann et al., 2020). This approach adjusted for missing data (Huque et al., 2018), which we had hypothesized may be missing especially for this sample with a range of functional skills and a majority with a known genetic etiology.
Broadly, we had hypothesized that we would see increased sensory behaviors related to clinical, functional, and genetic predictors. However, only one individual difference factor exhibited effects in this direction when considered as a single predictor: participants that were currently talking (i.e., at a minimum, phrase speech) exhibited increased seeking and sensitivity behaviors, consistent with other studies (Patten et al., 2013). However, other predictors did suggest increased sensory behaviors at the omnibus level (i.e., when all predictors were considered simultaneously). Individuals with an ID diagnosis exhibited poor registration and increased seeking behaviors. The extant literature on sensory processing in ASD and co-occurring ID includes contradictory findings (see review: Werkman et al., 2022). For instance, a prior study conflicted with the current results, such that there were no differences in either registration or seeking quadrants between ASD with ID diagnoses versus ID diagnosis alone, but there were differences to the other two quadrants (Joosten & Bundy, 2010). However, other work indicated children with lower nonverbal scores are implicated within a sensory phenotype that included hypo-responsivity (aligned with poor registration) and increased seeking (Liss et al., 2006). Relatedly, participants with a known genetic event exhibited poor registration behaviors, consistent with other genetic findings and described in more depth below for each subgroup.
It was surprising that our results indicated that having an ASD diagnosis was related to registration behaviors when considered with all predictors, such that participants with an ASD diagnosis were less likely to have registration behaviors (i.e., can notice sensory events). Because this sample is saturated for genetic etiologies, this finding may suggest that sensory behaviors are less pronounced when ASD diagnosis co-occurs with an ID diagnosis and/or a known genetic etiology. It is important to note the heterogeneity of this sample set that was ascertained in part due to a known ASD diagnosis (via the ZEBRA study) and otherwise for a known genetic etiology associated with neurodevelopmental disorders (via TIGER and BioGENE studies). Most participants (n = 178, 61.4%) had an ID diagnosis and had a known genetic etiology (n = 203, 70%), marking it as one of the largest studies to address sensory behaviors in both ID and genetic subgroups and subsequently extend large-scale work in ASD (Klintwall et al., 2011; Tillmann et al., 2020).
Sensory Profiles of Genetic Subgroups: ADNP, CHD8, DYRK1A, GRIN2B, and SCN2A
Although many genetics first studies have investigated clinical, medical, behavioral, and neural phenotypes, there is a limited understanding about whether specific profiles of sensory behaviors are associated with genetic etiologies. Here, we extended reports from case reports and small-scale studies to identify possible sensory phenotypes for five specific genetic subgroups that are the most common de novo mutations enriched genes in ASD (Iossifov et al., 2014): ADNP, CHD8, DYRK1A, GRIN2B, and SCN2A.
One notable pattern across these five groups was that all genetic subgroups are experiencing poor registration relative to the “idiopathic” ASD comparison group. Poor registration is described by Dunn (1997, 2007) as having a high neural threshold, such that stimuli need to be more intense to elicit behavioral response and individuals may otherwise may appear uninterested in sensory stimuli. As noted above, poor registration was also linked to ID diagnosis, which was more prevalent in the genetic subgroups. Prior to discussing specific sensory phenotypes and the implications for these subgroups, we note that this is counter to our predictions of high registration, based upon the animal and limited human physiological work relevant to each gene that predominantly indicated heightened sensory sensitivities (i.e., corresponding to high registration). The use of Dunn’s framework and caregiver reports may blunt interpretations by focusing on broad-based reports of behavior, as opposed to the nuance provided by neuroscience methods. Given the biological relevance of these subgroups, it will be critical to link sensory behaviors to neural processes in the future.
ADNP was characterized by poor registration and increased seeking behaviors, which was consistent with a mixed method study of children with ADNP mutations (Siper et al., 2021). Siper et al., (2021) observed strong seeking behaviors, including seeking of tactile (e.g., feeling textures, pressure) and auditory (e.g., using voice to make sounds outside of play) domains, in addition to hyporeactivity (consistent with poor registration) in 11 out of 22 ADNP participants. The two different ADNP classification leaves identified in our analysis may indicate a bimodal distribution based upon avoidance behaviors (see distribution presented in Fig. 2 violin plot).
When considering the CHD8 sensory phenotype, it is important to note that a majority of the CHD8 group (12 out of 21) was classified in a majority idiopathic ASD leaf. This may indicate that there are fewer differences between profiles for idiopathic ASD and CHD8. Prior animal models and limited human reports of auditory sensitivities implicated sensory sensitivities (Bernier et al., 2022; Morison et al., 2022). We would encourage future work to better specify and clarify domains of sensory interest. For instance, other models suggest a differential role of Dyrk1a expression within cerebellar nuclei that may be indicative of vestibular processing differences (Martı́ et al., 2003). Quantitative empirical studies would benefit from case presentation and qualitative summaries of sensory seeking behaviors to consider whether specific sensory interests are indeed thematically or behaviorally unique from autistic individuals.
Similar to other groups (ADNP, DYRK1A), the sensory phenotype for GRIN2B participants included poor registration and increased seeking. However, avoidance behaviors were an area in which the GRIN2B group was unique; specifically, the GRIN2B group was less likely to have avoidance behaviors that maps onto evidence of hyperactivity, impulsivity, and a strong desire for stimulation in GRIN2B (Freunscht et al., 2013). It may be the case that this phenotype is linked to an atypical attention phenotype also proposed given elevated prevalence of ADHD in GRIN2B (Dorval et al., 2007). In fact, new evidence implicates a role for one GRIN2B polymorphism (rs5796555-/A) for hyperactivity behaviors that are mediated by the density of the left isthmus of the cingulate cortex (Nobile et al., 2021). This region plays an important role in executive functions (Fornito et al., 2004) and preliminary evidence suggests cortical thickness may be distinct in autistic individuals (Hau et al., 2019). Therapeutic targets are currently being developed for GRIN2B to improve outcomes related to movement disorders and cortical visual impairment (see discussion in Platzer et al., 2017). Continued phenoty** work, particularly in regard to sensory behaviors, is timely and would complement therapeutic target development.
Lastly, SCN2A participants exhibited similar patterns as ADNP and GRIN2B participants in group comparisons, but the exploratory analysis indicated two diverging SCN2A classifications that were differentiated by sensitivity. One SCN2A classification group exhibited more frequent endorsement of sensitivities, in addition to poor registration (scores < 2.5), whereas the other exhibited minimal sensory issues aside from poor registration (scores ≥ 3.6). There is one prior case report of a 10-year-old child with a SCN2A mutation and multidimensional sensory atypicalities, including sensory seeking, poor registration, low endurance/tone, sedentary behavior, and distractibility (Tavassoli et al., 2014), which was partially consistent with these group results. It is important to note that the exploratory analysis model for sensitivity was poor for SCN2A, such that many were incorrectly characterized as idiopathic ASD. In addition, the majority of the SCN2A group were not currently talking (69.2%) and many were also non-ambulatory (33.8%), which may limit the ability for parents to assess sensory behaviors. As described earlier, this may be another subgroup in which a mixed methods approach (including neural correlates) will be an important avenue for future research (Uljarević et al., 2017).
Limitations
There are several limitations to note. First, when examining rare samples, such as participants with specific genetic etiologies, sample size is a common concern. Here, we sought to first examine relevant individual difference factors often observed in genetic subgroups, including clinical diagnosis and functional abilities, before directly comparing subgroups. However, there are additional medical and clinical co-occurring conditions that may be relevant. For instance, while prevalence rates of epilepsy in autistic individuals are varied, reported rates of seizures are greater than that in the general population (5–40%) (Canitano, 2007). Seizures may have clinical relevance to sensory processing, such that brain processes underlying seizure activity (i.e., increased neuronal connections) may also be indicated in hypersensitivity to sensory stimuli. While presence and prevalence of seizure activity is variable among the four targeted genetic subgroups discussed here, future work should consider characterizing participants with seizure activity to explore the relevance of additional individual difference factors in identifying unique sensory phenotypic profiles.
Relatedly, we acknowledge that it will be important to clarify the role of sensory modality by testing unimodal and possibly multimodal processing abilities. The current paper serves as a first step in unpacking individual differences within genetic subgroups that have heterogeneous clinical and functional presentations. While the Sensory Profile does provide a dimensional approach, additional future studies need to account for comprehensive, multidimensional, and multimodal assessment approaches across longitudinal time (see Uljarević et al., 2017). In addition, we encourage researchers to consider unpacking dynamic sensory phenotypes to understand sensory behaviors in real-time (for instance, neural habituation; see Hudac et al., 2017).
Second, it can be difficult to separate out codependent or shared aspects of sensory behaviors. The implementation of regression models aimed to optimize this assessment by including all individual difference factors within a single model for each measurement, before comparing the single regression coefficients. Yet, this statistical approach may insufficiently address aspects of different phenotypes that are truly codependent. As one example, diagnosis and walking statuses may collectively describe a unique profile, considering that walking milestones are delayed exponentially for children with lower nonverbal cognition scores (i.e., < 70; 31% in ASD, 60% in non-ASD) relative to children with higher scores (13% in ASD, 19% in non-ASD) (Bishop et al., 2016). Thus, these factors may be colinear in our models (and other research); a more detailed exploration would clarify how these individual difference factors may interact.
Conclusion
Our approach here presumed that sensory behaviors would be common among participants with a known genetic etiology. However, other aspects of the phenotype (e.g., medical history, sleep issues) within these subgroups as well as functional genetic differences may impact the variability observed in our results. For instance, while we clustered subgroups based upon a known gene that is disrupted, different variants may have different functional consequences. This is particularly notable for SCN2A, which has diverging phenotypes based upon whether the variant generates a loss or gain of function (Spratt et al., 2019). This level of granularity was beyond the scope of this paper but is essential for future work.
Notes
Please note that we have elected to use the term autism spectrum disorder (abbreviated to ASD) to describe certain subgroups throughout this manuscript, in part because our goal is to examine differences based upon the specific DSM-V diagnostic category. Recent guidance endorses the exclusive use of “identity-first” language (Dwyer 2022; Kenny et al., 2016). However, we note that language consensus is less clear for our sample that is uniquely saturated by presence of known genetic etiologies and co-occurring intellectual disability (> 60%). Thus, because our comparison group is unified in that the participants have an ASD diagnosis and do not have a known genetic etiology, we use the term “idiopathic ASD” to reference this group.
Here, we use the term ‘modulation’ based upon the definitions used by Dunn and colleagues in the Sensory Profile measure (W Dunn 1999) and subsequent publications (Ben-Sasson et al., 2007, 2009; Mulligan 2002). These sources describe sensory modulation as the regulation and management of one’s response to sensory input in a graded and adaptive manner.
References
Abrahams, B. S., Arking, D. E., Campbell, D. B., Mefford, H. C., Morrow, E. M., Weiss, L. A., et al. (2013). SFARI gene 2.0: a community-driven knowledgebase for the autism spectrum disorders (ASDs). Molecular Autism, 4(1), 36–36. https://doi.org/10.1186/2040-2392-4-36
Arnett, A. B., Rhoads, C. L., Hoekzema, K., Turner, T. N., Gerdts, J., Wallace, A. S., et al. (2018). The autism spectrum phenotype in ADNP syndrome. Autism Research, 11(9), 1300–1310. https://doi.org/10.1002/aur.1980
Association A P. (2013). Diagnostic and statistical manual of mental disorders. American Psychiatric Association. https://doi.org/10.1176/appi.books.9780890425596.dsm08
Baranek, G. T., Chin, Y. H., Hess, L. M. G., Yankee, J. G., Hatton, D. D., & Hooper, S. R. (2002). Sensory processing correlates of occupational performance in children with fragile X Syndrome: Preliminary findings. The American Journal of Occupational Therapy, 56(5), 538–546. https://doi.org/10.5014/ajot.56.5.538
Baranek, G. T., Woynaroski, T. G., Nowell, S., Turner-Brown, L., DuBay, M., Crais, E. R., & Watson, L. R. (2017). Cascading effects of attention disengagement and sensory seeking on social symptoms in a community sample of infants at-risk for a future diagnosis of autism spectrum disorder. Developmental Cognitive Neuroscience, 29, 30–40. https://doi.org/10.1016/j.dcn.2017.08.006
Barnard, R. A., Pomaville, M. B., & O’Roak, B. J. (2015). Mutations and modeling of the chromatin remodeler CHD8 define an emerging autism etiology. Frontiers in Neuroscience, 9, 477. https://doi.org/10.3389/fnins.2015.00477
Ben-Sasson, A., Cermak, S. A., Orsmond, G. I., Tager-Flusberg, H., Carter, A. S., Kadlec, M. B., & Dunn, W. (2007). Extreme sensory modulation behaviors in toddlers with autism spectrum disorders. The American Journal of Occupational Therapy, 61(5), 584–592. https://doi.org/10.5014/ajot.61.5.584
Ben-Sasson, A., Hen, L., Fluss, R., Cermak, S. A., Engel-Yeger, B., & Gal, E. (2009). A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(1), 1–11. https://doi.org/10.1007/s10803-008-0593-3
Bernier, R., Golzio, C., **ong, B., Stessman, H. A., Coe, B. P., Penn, O., et al. (2014). Disruptive CHD8 mutations define a subtype of autism early in development. Cell, 158(2), 263–276. https://doi.org/10.1016/j.cell.2014.06.017
Bishop, S. L., Thurm, A., Farmer, C., & Lord, C. (2016). Autism spectrum disorder, intellectual disability, and delayed walking. Pediatrics, 137(3), e20152959. https://doi.org/10.1542/peds.2015-2959
Bizzell, E., Ross, J., Rosenthal, C., Dumont, R., & Schaaf, R. (2020). Sensory features as a marker of autism spectrum disorders. Journal of Autism and Developmental Disorders, 50(6), 2240–2246. https://doi.org/10.1007/s10803-019-03948-8
Bon, Bregje W.M. van, Coe, B. P., Vries, B. B. de, & Eichler, E. E. (2021). DYRK1A Syndrome. The University of Washington, Seattle. https://www.ncbi.nlm.nih.gov/books/
Buyuktaskin, D., Iseri, E., Guney, E., Gunendi, Z., & Cengiz, B. (2021). Somatosensory temporal discrimination in autism spectrum disorder. Autism Research, 14(4), 656–667. https://doi.org/10.1002/aur.2479
Canitano, R. (2007). Epilepsy in autism spectrum disorders. European Child & Adolescent Psychiatry, 16(1), 61–66. https://doi.org/10.1007/s00787-006-0563-2
Chistol, L. T., Bandini, L. G., Must, A., Phillips, S., Cermak, S. A., & Curtin, C. (2018). Sensory sensitivity and food selectivity in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 48(2), 583–591. https://doi.org/10.1007/s10803-017-3340-9
Courcet, J.-B., Faivre, L., Malzac, P., Masurel-Paulet, A., Lopez, E., Callier, P., et al. (2012). The DYRK1A gene is a cause of syndromic intellectual disability with severe microcephaly and epilepsy. Journal of Medical Genetics, 49(12), 731. https://doi.org/10.1136/jmedgenet-2012-101251
Craney, T. A., & Surles, J. G. (2002). Model-dependent variance inflation factor cutoff values. Quality Engineering, 14(3), 391–403. https://doi.org/10.1081/qen-120001878
Delehanty, A. D., Stronach, S., Guthrie, W., Slate, E., & Wetherby, A. M. (2018). Verbal and nonverbal outcomes of toddlers with and without autism spectrum disorder, language delay, and global developmental delay. Autism & Developmental Language Impairments, 3, 2396941518764764. https://doi.org/10.1177/2396941518764764
Dellapiazza, F., Michelon, C., Vernhet, C., Muratori, F., Blanc, N., Picot, M.-C., et al. (2021). Sensory processing related to attention in children with ASD, ADHD, or typical development: Results from the ELENA cohort. European Child & Adolescent Psychiatry, 30(2), 283–291. https://doi.org/10.1007/s00787-020-01516-5
Dorval, K. M., Wigg, K. G., Crosbie, J., Tannock, R., Kennedy, J. L., Ickowicz, A., et al. (2007). Association of the glutamate receptor subunit gene GRIN2B with attention-deficit/hyperactivity disorder. Genes, Brain and Behavior, 6(5), 444–452. https://doi.org/10.1111/j.1601-183x.2006.00273.x
Dunn, W. (1997). The impact of sensory processing abilities on the daily lives of young children and their families: A conceptual model. Infants & Young Children, 9(4), 23–35. https://doi.org/10.1097/00001163-199704000-00005
Dunn, W. (1999). Sensory profile: Users manual. San Antonio.
Dunn, W. (2007). Supporting children to participate successfully in everyday life by using sensory processing knowledge. Infants & Young Children, 20(2), 84–101. https://doi.org/10.1097/01.iyc.0000264477.05076.5d
Dunn, W., & Ermer, J. (1998). The sensory profile: A discriminant analysis of children with and without disabilities. American Journal of Occupational Therapy, 4(52), 283–290.
Dwyer, P. (2022). Stigma, incommensurability, or both? Pathology-first, person-first, and identity-first language and the challenges of discourse in divided autism communities. Journal of Developmental & Behavioral Pediatrics, 43(2), 111–113. https://doi.org/10.1097/dbp.0000000000001054
Earl, R. K., Turner, T. N., Mefford, H. C., Hudac, C. M., Gerdts, J., Eichler, E. E., & Bernier, R. A. (2017). Clinical phenotype of ASD-associated DYRK1A haploinsufficiency. Molecular Autism. https://doi.org/10.1186/s13229-017-0173-5
Eaton, M., Zhang, J., Ma, Z., Park, A. C., Lietzke, E., Romero, C. M., et al. (2021). Generation and basic characterization of a gene-trap knockout mouse model of Scn2a with a substantial reduction of voltage-gated sodium channel Nav1.2 expression. Genes Brain and Behavior, 20(4), e12725. https://doi.org/10.1111/gbb.12725
Fenster, R., Ziegler, A., Kentros, C., Geltzeiler, A., Snyder, L. G., Brooks, E., & Chung, W. K. (2022). Characterization of phenotypic range in DYRK1A haploinsufficiency syndrome using standardized behavioral measures. American Journal of Medical Genetics Part A, 188(7), 1954–1963. https://doi.org/10.1002/ajmg.a.62721
Fischbach, G. D., & Lord, C. (2010). The Simons simplex collection: A resource for identification of autism genetic risk factors. Neuron, 68(2), 192–195. https://doi.org/10.1016/j.neuron.2010.10.006
Fornito, A., Yücel, M., Wood, S., Stuart, G. W., Buchanan, J.-A., Proffitt, T., et al. (2004). Individual differences in anterior cingulate/paracingulate morphology are related to executive functions in healthy males. Cerebral Cortex, 14(4), 424–431. https://doi.org/10.1093/cercor/bhh004
Fotaki, V., Dierssen, M., Alcántara, S., Martínez, S., Martí, E., Casas, C., et al. (2002). Dyrk1A haploinsufficiency affects viability and causes developmental delay and abnormal brain morphology in mice. Molecular and Cellular Biology, 22(18), 6636–6647. https://doi.org/10.1128/mcb.22.18.6636-6647.2002
Freunscht, I., Popp, B., Blank, R., Endele, S., Moog, U., Petri, H., et al. (2013). Behavioral phenotype in five individuals with de novo mutations within the GRIN2B gene. Behavioral and Brain Functions, 9(1), 20. https://doi.org/10.1186/1744-9081-9-20
Gozes, I., Dijck, A. V., Hacohen-Kleiman, G., Grigg, I., Karmon, G., Giladi, E., et al. (2017). Premature primary tooth eruption in cognitive/motor-delayed ADNP-mutated children. Translational Psychiatry, 7(2), e1043. https://doi.org/10.1038/tp.2017.27
Grzadzinski, R., Donovan, K., Truong, K., Nowell, S., Lee, H., Sideris, J., et al. (2020). Sensory reactivity at 1 and 2 years old is associated with ASD severity during the preschool years. Journal of Autism and Developmental Disorders, 50(11), 3895–3904. https://doi.org/10.1007/s10803-020-04432-4
Hau, J., Aljawad, S., Baggett, N., Fishman, I., Carper, R. A., & Müller, R. (2019). The cingulum and cingulate U-fibers in children and adolescents with autism spectrum disorders. Human Brain Map**, 40(11), 3153–3164. https://doi.org/10.1002/hbm.24586
Helsmoortel, C., Silfhout, A. T. V., Coe, B. P., Vandeweyer, G., Rooms, L., van den Ende, J., et al. (2014). A SWI/SNF related autism syndrome caused by de novo mutations in ADNP. Nature Genetics, 46(4), 380–384. https://doi.org/10.1038/ng.2899
Hu, C., Chen, W., Myers, S. J., Yuan, H., & Traynelis, S. F. (2016). Human GRIN2B variants in neurodevelopmental disorders. Journal of Pharmacological Sciences, 132(2), 115–121. https://doi.org/10.1016/j.jphs.2016.10.002
Hudac, C. M., Bove, J., Barber, S., Duyzend, M., Wallace, A., Martin, C. L., et al. (2020). Evaluating heterogeneity in ASD symptomatology, cognitive ability, and adaptive functioning among 16p11.2 CNV carriers. Autism Research. https://doi.org/10.1002/aur.2332
Hudac, C. M., Stessman, H. A. F., DesChamps, T. D., Kresse, A., Faja, S., Neuhaus, E., et al. (2017). Exploring the heterogeneity of neural social indices for genetically distinct etiologies of autism. Journal of Neurodevelopmental Disorders, 9(1), 24. https://doi.org/10.1186/s11689-017-9199-4
Huque, M. H., Carlin, J. B., Simpson, J. A., & Lee, K. J. (2018). A comparison of multiple imputation methods for missing data in longitudinal studies. BMC Medical Research Methodology, 18(1), 168. https://doi.org/10.1186/s12874-018-0615-6
Iossifov, I., O’Roak, B. J., Sanders, S. J., Ronemus, M., Krumm, N., Levy, D., et al. (2014). The contribution of de novo coding mutations to autism spectrum disorder. Nature, 515(7526), 216–221. https://doi.org/10.1038/nature13908
Iossifov, I., Ronemus, M., Levy, D., Wang, Z., Hakker, I., Rosenbaum, J., et al. (2012). De novo gene disruptions in children on the autistic spectrum. Neuron, 74(2), 285–299. https://doi.org/10.1016/j.neuron.2012.04.009
Joosten, A. V., & Bundy, A. C. (2010). Sensory processing and stereotypical and repetitive behaviour in children with autism and intellectual disability. Australian Occupational Therapy Journal, 57(6), 366–372. https://doi.org/10.1111/j.1440-1630.2009.00835.x
Kaat, A. J., Shui, A. M., Ghods, S. S., Farmer, C. A., Esler, A. N., Thurm, A., et al. (2021). Sex differences in scores on standardized measures of autism symptoms: A multisite integrative data analysis. Journal of Child Psychology and Psychiatry, 62(1), 97–106. https://doi.org/10.1111/jcpp.13242
Kapp, S. K. (2013). Empathizing with sensory and movement differences: Moving toward sensitive understanding of autism. Frontiers in Integrative Neuroscience, 7, 38. https://doi.org/10.3389/fnint.2013.00038
Kaufman, L., Ayub, M., & Vincent, J. B. (2010). The genetic basis of non-syndromic intellectual disability: A review. Journal of Neurodevelopmental Disorders, 2(4), 182–209. https://doi.org/10.1007/s11689-010-9055-2
Kenny, L., Hattersley, C., Molins, B., Buckley, C., Povey, C., & Pellicano, E. (2016). Which terms should be used to describe autism? Perspectives from the UK autism community. Autism, 20(4), 442–462. https://doi.org/10.1177/1362361315588200
Kern, J. K., Garver, C. R., Carmody, T., Andrews, A. A., Trivedi, M. H., & Mehta, J. A. (2007). Examining sensory quadrants in autism. Research in Autism Spectrum Disorders, 1(2), 185–193. https://doi.org/10.1016/j.rasd.2006.09.002
Kim, O.-H., Cho, H.-J., Han, E., Hong, T. I., Ariyasiri, K., Choi, J.-H., et al. (2017). Zebrafish knockout of down syndrome gene, DYRK1A, shows social impairments relevant to autism. Molecular Autism, 8(1), 50. https://doi.org/10.1186/s13229-017-0168-2
Klintwall, L., Holm, A., Eriksson, M., Carlsson, L. H., Olsson, M. B., Hedvall, Å., et al. (2011). Sensory abnormalities in autism a brief report. Research in Developmental Disabilities, 32(2), 795–800. https://doi.org/10.1016/j.ridd.2010.10.021
Krumm, N., O’Roak, B. J., Shendure, J., & Eichler, E. E. (2014). A de novo convergence of autism genetics and molecular neuroscience. Trends in Neurosciences, 37(2), 95–105. https://doi.org/10.1016/j.tins.2013.11.005
Kurtz-Nelson, E. C., Tham, S. W., Ahlers, K., Cho, D., Wallace, A. S., Eichler, E. E., et al. (2021). Brief Report: Associations between self-injurious behaviors and abdominal pain among individuals with ASD-associated disruptive mutations. Journal of Autism and Developmental Disorders, 51(9), 3365–3373. https://doi.org/10.1007/s10803-020-04774-z
la Marche, W. D., Steyaert, J., & Noens, I. (2012). Atypical sensory processing in adolescents with an autism spectrum disorder and their non-affected siblings. Research in Autism Spectrum Disorders, 6(2), 639–645. https://doi.org/10.1016/j.rasd.2011.09.014
Lane, A. E., Young, R. L., Baker, A. E. Z., & Angley, M. T. (2010). Sensory processing subtypes in autism: Association with adaptive behavior. Journal of Autism and Developmental Disorders, 40(1), 112–122. https://doi.org/10.1007/s10803-009-0840-2
Laton, J. (2020). Convenience layer for cross-validation in R. github.com. https://github.com/jlaton/classification
Laton, J., Schependom, J. V., Gielen, J., Decoster, J., Moons, T., Keyser, J. D., et al. (2014). Single-subject classification of schizophrenia patients based on a combination of oddball and mismatch evoked potential paradigms. Journal of the Neurological Sciences, 347(1–2), 262–267. https://doi.org/10.1016/j.jns.2014.10.015
Lavenne-Collot, N., Jallot, N., Maguet, J., Degrez, C., Botbol, M., & Grandgeorge, M. (2021). Early motor skills in children with autism spectrum disorders are marked by less frequent hand and knees crawling. Perceptual and Motor Skills, 128(5), 2148–2165. https://doi.org/10.1177/00315125211037983
Lee, K.-S., Choi, M., Kwon, D.-W., Kim, D., Choi, J.-M., Kim, A.-K., et al. (2020). A novel de novo heterozygous DYRK1A mutation causes complete loss of DYRK1A function and developmental delay. Scientific Reports, 10(1), 9849. https://doi.org/10.1038/s41598-020-66750-y
Léna, I., & Mantegazza, M. (2019). NaV1.2 haploinsufficiency in Scn2a knock-out mice causes an autistic-like phenotype attenuated with age. Scientific Reports, 9(1), 12886. https://doi.org/10.1038/s41598-019-49392-7
Lippert, C., Quon, G., Kang, E. Y., Kadie, C. M., Listgarten, J., & Heckerman, D. (2013). The benefits of selecting phenotype-specific variants for applications of mixed models in genomics. Scientific Reports, 3(1), 1815. https://doi.org/10.1038/srep01815
Liss, M., Saulnier, C., Fein, D., & Kinsbourne, M. (2006). Sensory and attention abnormalities in autistic spectrum disorders. Autism, 10(2), 155–172. https://doi.org/10.1177/1362361306062021
Little, L. M., Ausderau, K., Sideris, J., & Baranek, G. T. (2015). Activity participation and sensory features among children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(9), 2981–2990. https://doi.org/10.1007/s10803-015-2460-3
Loh, S. Y., Ee, S. I., & Marret, M. J. (2021). Sensory processing and its relationship to participation among childhood occupations in children with autism spectrum disorder: Exploring the profile of differences. International Journal of Developmental Disabilities. https://doi.org/10.1080/20473869.2021.1950484
Mart, E., Altafaj, X., Dierssen, M., de la Luna, S., Fotaki, V., Alvarez, M., Pérez-Riba, M., Ferrer, I., & Estivill, X. (2003). Dyrk1A expression pattern supports specific roles of this kinase in the adult central nervous system. Brain Research, 964(2), 250–263. https://doi.org/10.1016/s0006-8993(02)04069-6
McCarthy, S. E., Gillis, J., Kramer, M., Lihm, J., Yoon, S., Berstein, Y., et al. (2014). De novo mutations in schizophrenia implicate chromatin remodeling and support a genetic overlap with autism and intellectual disability. Molecular Psychiatry, 19(6), 652–658. https://doi.org/10.1038/mp.2014.29
Mishra, N., Kouzmitcheva, E., Orsino, A., & Minassian, B. (2016). Chromosome 12p deletion spanning the GRIN2B gene presenting with a neurodevelopmental phenotype: A case report and review of literature. Child neurology open, 3, 2329048X16629980. https://doi.org/10.1177/2329048x16629980
Möhrle, D., Fernández, M., Peñagarikano, O., Frick, A., Allman, B., & Schmid, S. (2019). What we can learn from a genetic rodent model about autism. Neuroscience & Biobehavioral Reviews, 109, 29–53. https://doi.org/10.1016/j.neubiorev.2019.12.015
Monyer, H., Sprengel, R., Schoepfer, R., Herb, A., Higuchi, M., Lomeli, H., et al. (1992). Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science, 256(5060), 1217–1221. https://doi.org/10.1126/science.256.5060.1217
Morison, L. D., Braden, R. O., Amor, D. J., Brignell, A., van Bon, B. W. M., & Morgan, A. T. (2022). Social motivation a relative strength in DYRK1A syndrome on a background of significant speech and language impairments. European Journal of Human Genetics. https://doi.org/10.1038/s41431-022-01079-w
Mulligan, S. (2002). Advances in sensory integration research. In A. C. Bundy, S. J. Lne, & E. A Murray (Eds.), Sensory integration: Theory and practice (Vol. 2nd ed., pp. 397–411).
Nobile, M., Maggioni, E., Mauri, M., Garzitto, M., Piccin, S., Bonivento, C., et al. (2021). Brain anatomical mediators of GRIN2B gene association with attention/hyperactivity problems: An integrated genetic-neuroimaging study. Genes, 12(8), 1193. https://doi.org/10.3390/genes12081193
Patten, E., Ausderau, K. K., Watson, L. R., & Baranek, G. T. (2013). Sensory response patterns in nonverbal children with ASD. Autism Research and Treatment, 2013, 436286. https://doi.org/10.1155/2013/436286
Platzer, K., Yuan, H., Schütz, H., Winschel, A., Chen, W., Hu, C., et al. (2017). GRIN2B encephalopathy: Novel findings on phenotype, variant clustering, functional consequences and treatment aspects. Journal of Medical Genetics, 54(7), 460. https://doi.org/10.1136/jmedgenet-2016-104509
Roberts, T. F., Gobes, S. M. H., Murugan, M., Ölveczky, B. P., & Mooney, R. (2012). Motor circuits are required to encode a sensory model for imitative learning. Nature Neuroscience, 15(10), 1454–1459. https://doi.org/10.1038/nn.3206
Robinson, E. B., Lichtenstein, P., Anckarsäter, H., Happé, F., & Ronald, A. (2013). Examining and interpreting the female protective effect against autistic behavior. Proceedings of the National Academy of Sciences, 110(13), 5258–5262. https://doi.org/10.1073/pnas.1211070110
Sanders, S. J., Campbell, A. J., Cottrell, J. R., Møller, R. S., Wagner, F. F., Auldridge, A. L., et al. (2018). Progress in understanding and treating SCN2A-mediated disorders. Trends in Neurosciences, 41(7), 1–15. https://doi.org/10.1016/j.tins.2018.03.011
Siper, P. M., Layton, C., Levy, T., Lurie, S., Benrey, N., Zweifach, J., et al. (2021). Sensory reactivity symptoms are a core feature of ADNP syndrome irrespective of autism diagnosis. Genes, 12(3), 351. https://doi.org/10.3390/genes12030351
Smith, A. M., Roux, S., Naidoo, N. T., & (Raj), & Venter, D. J. L. (2005). Food choices of tactile defensive children. Nutrition, 21(1), 14–19. https://doi.org/10.1016/j.nut.2004.09.004
Spratt, P. W. E., Ben-Shalom, R., Keeshen, C. M., Burke, K. J., Clarkson, R. L., Sanders, S. J., & Bender, K. J. (2019). The autism-associated gene Scn2a contributes to dendritic excitability and synaptic function in the prefrontal cortex. Neuron, 103(4), 673-685.e5. https://doi.org/10.1016/j.neuron.2019.05.037
Stessman, H. A., Bernier, R., & Eichler, E. E. (2014). A genotype-first approach to defining the subtypes of a complex disease. Cell, 156(5), 872–877. https://doi.org/10.1016/j.cell.2014.02.002
Suetterlin, P., Hurley, S., Mohan, C., Riegman, K. L. H., Pagani, M., Caruso, A., et al. (2018). Altered neocortical gene expression, brain overgrowth and functional over-connectivity in Chd8 haploinsufficient mice. Cerebral cortex, 28(6), 2192–2206. https://doi.org/10.1093/cercor/bhy058
Takahashi, H., Nakahachi, T., Stickley, A., Ishitobi, M., & Kamio, Y. (2018). Relationship between physiological and parent-observed auditory over-responsiveness in children with typical development and those with autism spectrum disorders. Autism, 22(3), 291–298. https://doi.org/10.1177/1362361316680497
Talay-Ongan, A., & Wood, K. (2000). Unusual sensory sensitivities in autism: A possible crossroads. International Journal of Disability, Development and Education, 47(2), 201–212. https://doi.org/10.1080/713671112
Tatsukawa, T., Raveau, M., Ogiwara, I., Hattori, S., Miyamoto, H., Mazaki, E., et al. (2019). Scn2a haploinsufficient mice display a spectrum of phenotypes affecting anxiety, sociability, memory flexibility and ampakine CX516 rescues their hyperactivity. Molecular Autism, 10(1), 15. https://doi.org/10.1186/s13229-019-0265-5
Tavassoli, T., Kolevzon, A., Wang, A. T., Curchack-Lichtin, J., Halpern, D., Schwartz, L., et al. (2014). De novo SCN2A splice site mutation in a boy with Autism spectrum disorder. BMC Medical Genetics, 15(1), 1–8. https://doi.org/10.1186/1471-2350-15-35
Tejedor, F., Zhu, X. R., Kaltenbach, E., Ackermann, A., Baumann, A., Canal, I., et al. (1995). minibrain: A new protein kinase family involved in postembryonic neurogenesis in Drosophila. Neuron, 14(2), 287–301. https://doi.org/10.1016/0896-6273(95)90286-4
Therneau, T., & Atkinson, B. (2019). rpart: Recursive Partitioning and Regression Trees. R package version 4.1.-10. https://CRAN.R-project.org/package=rpart
Thompson, B. A., Tremblay, V., Lin, G., & Bochar, D. A. (2008). CHD8 is an ATP-dependent chromatin remodeling factor that regulates beta-catenin target genes. Molecular and cellular biology, 28(12), 3894–3904. https://doi.org/10.1128/mcb.00322-08
Tillmann, J., Uljarevic, M., Crawley, D., Dumas, G., Loth, E., Murphy, D., et al. (2020). Dissecting the phenotypic heterogeneity in sensory features in autism spectrum disorder: A factor mixture modelling approach. Molecular Autism, 11(1), 67. https://doi.org/10.1186/s13229-020-00367-w
Tomchek, S. D., & Dunn, W. (2007). Sensory processing in children with and without autism: A comparative study using the short sensory profile. Am J Occup Ther, 61(2), 190–200.
Tomchek, S. D., Huebner, R. A., & Dunn, W. (2014). Patterns of sensory processing in children with an autism spectrum disorder. Research in Autism Spectrum Disorders, 8(9), 1214–1224. https://doi.org/10.1016/j.rasd.2014.06.006
Tzischinsky, O., Meiri, G., Manelis, L., Bar-Sinai, A., Flusser, H., Michaelovski, A., et al. (2018). Sleep disturbances are associated with specific sensory sensitivities in children with autism. Molecular Autism, 9(1), 22. https://doi.org/10.1186/s13229-018-0206-8
Uljarević, M., Baranek, G., Vivanti, G., Hedley, D., Hudry, K., & Lane, A. (2017). Heterogeneity of sensory features in autism spectrum disorder: Challenges and perspectives for future research. Autism Research, 10(5), 703–710. https://doi.org/10.1002/aur.1747
Uljarević, M., Prior, M. R., & Leekam, S. R. (2014). First evidence of sensory atypicality in mothers of children with autism spectrum disorder (ASD). Molecular Autism, 5(1), 26–26. https://doi.org/10.1186/2040-2392-5-26
van Bon, B. W. M., Coe, B. P., Bernier, R., Green, C., Gerdts, J., Witherspoon, K., et al. (2016). Disruptive de novo mutations of DYRK1A lead to a syndromic form of autism and ID. Molecular Psychiatry, 21(1), 126–132. https://doi.org/10.1038/mp.2015.5
van Bon, B., Hoischen, A., Hehir-Kwa, J., de Brouwer, A., Ruivenkamp, C., Gijsbers, A., et al. (2011). Intragenic deletion in DYRK1A leads to mental retardation and primary microcephaly. Clinical genetics, 79(3), 296–299. https://doi.org/10.1111/j.1399-0004.2010.01544.x
von Engelhardt, J., Doganci, B., Jensen, V., Hvalby, Ø., Göngrich, C., Taylor, A., et al. (2008). Contribution of hippocampal and extra-hippocampal NR2B-Containing NMDA receptors to performance on spatial learning tasks. Neuron, 60(5), 846–860. https://doi.org/10.1016/j.neuron.2008.09.039
Wang, C.-C., Held, R. G., Chang, S.-C., Yang, L., Delpire, E., Ghosh, A., & Hall, B. J. (2011). A critical role for GluN2B-containing NMDA receptors in cortical development and function. Neuron, 72(5), 789–805. https://doi.org/10.1016/j.neuron.2011.09.023
Werkman, M. F., Landsman, J. A., Fokkens, A. S., Dijkxhoorn, Y. M., van Berckelaer-Onnes, I. A., Begeer, S., & Reijneveld, S. A. (2022). The impact of the presence of intellectual disabilities on sensory processing and behavioral outcomes among individuals with autism spectrum disorders: a systematic review. Review Journal of Autism and Developmental Disorders. https://doi.org/10.1007/s40489-022-00301-1
Widowati, E. W., Ernst, S., Hausmann, R., Müller-Newen, G., & Becker, W. (2018). Functional characterization of DYRK1A missense variants associated with a syndromic form of intellectual deficiency and autism. Biology Open, 7(4), bio032862. https://doi.org/10.1242/bio.032862
Williams, K. L., Kirby, A. V., Watson, L. R., Sideris, J., Bulluck, J., & Baranek, G. T. (2018). Sensory features as predictors of adaptive behaviors: A comparative longitudinal study of children with autism spectrum disorder and other developmental disabilities. Research in Developmental Disabilities, 81, 103–112. https://doi.org/10.1016/j.ridd.2018.07.002
Wodka, E. L., Mathy, P., & Kalb, L. (2013). Predictors of phrase and fluent speech in children with autism and severe language delay. Pediatrics, 131(4), e1128–e1134. https://doi.org/10.1542/peds.2012-2221
Wolff, M., Johannesen, K. M., Hedrich, U. B. S., Masnada, S., Rubboli, G., Gardella, E., et al. (2017). Genetic and phenotypic heterogeneity suggest therapeutic implications in SCN2A-related disorders. Brain, 140(5), 1316–1336. https://doi.org/10.1093/brain/awx054
Yamagata, T., Ogiwara, I., Mazaki, E., Yanagawa, Y., & Yamakawa, K. (2017). Nav1.2 is expressed in caudal ganglionic eminence-derived disinhibitory interneurons: Mutually exclusive distributions of Nav1.1 and Nav1.2. Biochemical and Biophysical Research Communications, 491(4), 1070–1076. https://doi.org/10.1016/j.bbrc.2017.08.013
Yang, J., Zaitlen, N. A., Goddard, M. E., Visscher, P. M., & Price, A. L. (2014). Advantages and pitfalls in the application of mixed-model association methods. Nature Genetics, 46(2), 100–106. https://doi.org/10.1038/ng.2876
Acknowledgments
We are grateful to the families and participants of this study who completed the 125 questions on the Sensory Profile and thousands of other questions. The authors also thank Dr. Anne Arnett for her support in develo** the analytical strategy for classification. This work was supported by grants from the FamiliesSCN2A Foundation (2019 Action Potential Grant to C.M.H.), the GRIN2B Foundation (2019 Small Grant Award to C.M.H.), and the National Institutes for Mental Health (R01 MH100047 to R.A.B., R01 MH101221 to E.E.E.). The data that support the findings of this study are in part available from the NIMH Data Archive for the TIGER and ZEBRA studies. The remaining data from the BioGENE Study are available from the corresponding author, C.M.H., upon reasonable request. E.E.E. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
Conceptualization, Methodology, Formal analysis and investigation: Dr. Caitlin Hudac; Writing - original draft preparation: Dr. Caitlin Hudac, Nicole Friedman, Victoria Ward, Rachel Estreicher, Grace Dorsey; Writing - review and editing: All authors; Funding acquisition: Dr. Caitlin Hudac, Dr. Raphael Bernier, Dr. Evan Eichler.
Corresponding author
Ethics declarations
Conflicts of interest
C.M.H. is a scientific advisory board member for FamiliesSCN2A Foundation. E.E.E. is a scientific advisory board member of Variant Bio, Inc. No other authors declare any conflicts.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hudac, C.M., Friedman, N.R., Ward, V.R. et al. Characterizing Sensory Phenotypes of Subgroups with a Known Genetic Etiology Pertaining to Diagnoses of Autism Spectrum Disorder and Intellectual Disability. J Autism Dev Disord 54, 2386–2401 (2024). https://doi.org/10.1007/s10803-023-05897-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-023-05897-9