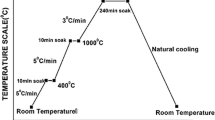
The sintering kinetics of periclase with brucite-aluminum phosphate binder was studied. A model that takes into account the role of the physical compaction and chemical binding in the presence of a binder during the heating process was used to determine the kinetic parameters. The strength of a conglomerate was due to the sintering itself as well as the action of the binder. The effective energy of activation of the sintering of periclase was determined: Ea = 287 ± 9 kJ/mole, which is close to the activation energy of oxygen diffusion in MgO. Spinel MgAl2O4, which slows the sintering process, forms in the material at high temperatures.



Similar content being viewed by others
References
S. L. Golynko-Wolfson, M. M. Sychev, and L. G. Sudakas, Chemical Principles of the Technology and Application of Phosphate Bonds and Coatings [in Russian], Khimiya, Leningrad (1968).
L. G. Sudakas, Phosphate Binding Systems [in Russian], RIA Kvintet, St. Petersburg (2006).
A. P. Luz, D. T. Gomes, and V. C. Pandolfelli, “High-alumina phosphate-bonded refractory castables: Al(OH)3 sources and their effects,” Ceram. Int., 41, 9041 – 9050 (2015).
A. Viani, K. Sotiriadis, P. Sasek, and V.-S. Appoavou, “Evolution of microstructure and performance in magnesium potassium phosphate ceramics: role of sintering temperature of MgO powder,” Ceram. Int., 42, 16310 – 16316 (2016).
V. A. Abyzov, “Lightweight refractory concrete based on aluminum- magnesium-phosphate binder,” Proc. Eng., 150, 1440 – 1445 (2016).
Lv, F. T. Wang, L. G. Wang, and E. Ze, “The preparation of phosphate bonding agent and its application in ceramic coating,” Adv. Mat. Res., 821 – 822, 1256 – 1260 (2013).
I. E. Illarionov and I. A. Strelnikov, “Thermal insulation metal phosphate mixtures and methods of their application in foundry production,” Teoriya Tekhnol. Metallurg. Proiz-va, 20(1), 27 – 30 (2017).
I. D. Kashcheeva (ed.), Handbook of Refractories for Industrial Units and Furnaces, Vol. 1, Production of Refractory Materials [in Russian], Intermet Inginiring, Moscow (2000).
D. Mohapatra and D. Sarkar, “Preparation of MgO–MgAl2O4 composite for refractory application,” J. Mater. Proc. Technol., 189(1 – 3), 279 – 283 (2007).
E. M. M. Ewais, A. Elamir, D. Besisa, et al., “Synthesis of nanocrystalline MgO/MgAl2O4 spinel powders from industrial wastes,” J. Alloys Compd., 691, 822 – 833 (2017).
N. F. Kosenko, N. V. Filatova, and M. A. Glazkov, “Brucite-based magnesium phosphate bonding agent, its analysis and application for periclase sintering,” Izv. Vyssh. Uchebn. Zaved., Khim. Khimich. Tekhnol., 6(12), 119 – 124 (2019).
B. R. Vahid and M. Haghighi, “Thermochemical synthesis of Mg–Al ceramic spinel as support for MgO/MgAl2O4 nanocatalyst toward conversion of vegetable oil to green fuel,” Petrol. Res., 28(102), 21 – 23 (2018).
L. B. Horoshavin, V. A. Perepelitsyn, and V. A. Kononov, Handbook of Magnesia Refractories [in Russian], Intermet Inginiring, Moscow (2001).
N. F. Kosenko, N. V. Filatova, and O. P. Denisova, “Simulation of the isothermal sintering process for corundum materials on a chemical bond,” Izv. Vyssh. Uchebn. Zaved., Khim. Khimich. Tekhnol., 47(7), 113 – 116 (2004).
G. V. Samsonov (ed.), Handbook of the Physicochemical Properties of Oxides [in Russian], Metallurgiya, Moscow (1978).
S. V. Sinel’nikov, V. M. Gropyanov, and V. G. Abakumov, “Kinetics of nonisothermal sintering of magnesium oxide,” J. Appl. Chem., 55(4), 765 – 769 (1982).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 9, pp. 16 – 20, September, 2020.
Rights and permissions
About this article
Cite this article
Filatova, N.V., Kosenko, N.F. & Glazkov, M.A. Sintering of Periclase with Brucite-Aluminum Phosphate Binder. Glass Ceram 77, 340–343 (2021). https://doi.org/10.1007/s10717-021-00303-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-021-00303-1