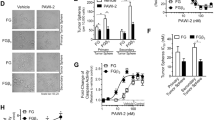
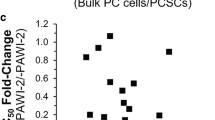
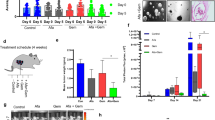
Abstract
Worldwide, pancreatic cancer (PC) is a major health problem and almost 0.5 million people were diagnosed with PC in 2020. In the United States, more than 64,000 adults will be diagnosed with PC in 2023. PC is highly resistant to currently available treatments and standard of care chemotherapies cause serious side effects. Most PC patients are resistant to clinical therapies. Combination therapy has showed superior efficacy over single-agent treatment. However, most therapy has failed to show a significant improvement in overall survival due to treatment-related toxicity. Develo** efficacious clinically useful PC therapies remains a challenge. Herein, we show the efficacy of an innovative pathway modulator, p53-Activator Wnt Inhibitor-2 (PAWI-2) against tumors arising from human pancreatic cancer stem cells (i.e., hPCSCs, FGβ3 cells). PAWI-2 is a potent inhibitor of tumor growth. In the present study, we showed PAWI-2 potently inhibited growth of tumors from hPCSCs in orthopic xenograft models of both male and female mice. PAWI-2 worked in a non-toxic manner to inhibit tumors. Compared to vehicle-treated animals, PAWI-2 modulated molecular regulators of tumors. Anti-cancer results showed PAWI-2 in vivo efficacy could be correlated to in vitro potency to inhibit FGβ3 cells. PAWI-2 represents a safe, new approach to combat PC.

Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
Code availability
No datasets were generated or analysed during the current study.
References
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74(11):2913–2921. https://doi.org/10.1158/0008-5472.CAN-14-0155
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30. https://doi.org/10.3322/caac.21387
Li D, **e K, Wolff R, Abbruzzese JL (2004) Pancreatic cancer. Lancet. 363(9414):1049–1057. https://doi.org/10.1016/S0140-6736(04)15841-8
Ansari D, Tingstedt B, Andersson B, Holmquist F, Sturesson C, Williamsson C et al (2016) Pancreatic cancer: yesterday, today and tomorrow. Future Oncol 12(16):1929–1946. https://doi.org/10.2217/fon-2016-0010
National Cancer Institute at the National Institutes of Health (2020) Drugs Approved for Pancreatic Cancer. https://www.cancer.gov/about-cancer/treatment/drugs/pancreatic
Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6):2403–2413. https://doi.org/10.1200/jco.1997.15.6.2403
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825. https://doi.org/10.1056/NEJMoa1011923
** J, Teng C, Li T (2018) Combination therapy versus gemcitabine monotherapy in the treatment of elderly pancreatic cancer: a meta-analysis of randomized controlled trials. Drug Des Devel Ther 12:475–480. https://doi.org/10.2147/DDDT.S156766
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25(15):1960–1966. https://doi.org/10.1200/JCO.2006.07.9525
Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C et al (2008) Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomized phase II study. Lancet 371(9630):2101–2108. https://doi.org/10.1016/S0140-6736(08)60661-3
Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ et al (2010) Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 28(22):3605–3610. https://doi.org/10.1200/JCO.2009.25.7550
Kreso A, Dick JE (2014) Evolution of the cancer stem cell model. Cell Stem Cell 14:275–291. https://doi.org/10.1016/j.stem.2014.02.006
Nassar D, Blanpain C (2016) Cancer Stem cells: Basic concepts and therapeutic implications. Annu Rev Pathol 11:47–76. https://doi.org/10.1146/annurev-pathol-012615-044438
Dean M, Fojo T, Bates S (2005) Tumor stem cells and drug resistance. Nat Rev Cancer 5:275–284. https://doi.org/10.1038/nrc1590
Hermann PC et al (2007) Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1:313–323. https://doi.org/10.1016/j.stem.2007.06.002
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Meacham CE, Morrison SJ (2013) Tumor heterogeneity and cancer cell plasticity. Nature 501:328–337. https://doi.org/10.1038/nature12624
Bivona TG et al (2011) FAS and NF-kappaB signalling modulate dependence of lung cancers on mutant EGFR. Nature 471:523–526. https://doi.org/10.1038/nature09870
Seguin L et al (2014) An integrin beta(3)-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol 16:457–468. https://doi.org/10.1038/ncb2953
Desgrosellier JS et al (2009) An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med 15:1163–1169. https://doi.org/10.1038/nm.2009
Seguin L et al (2017) Galectin-3, a Druggable vulnerability for KRAS-Addicted cancers. Cancer Discov 7:1464–1479. https://doi.org/10.1158/2159-8290.CD-17-0539
Desgrosellier JS, Cheresh DA (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10:9–22. https://doi.org/10.1038/nrc2748
Adhikari AS, Agarwal N, Iwakuma T (2011) Metastatic potential of tumor-initiating cells in solid tumors. Front Biosci 16:1927–1938
Miller PG et al (2013) In vivo RNAi screening identifies a leukemia-specific dependence on integrin beta 3 signaling. Cancer Cell 24:45–58. https://doi.org/10.1016/j.ccr.2013.05.004
Vaillant F et al (2008) The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammarytumorigenesis. Cancer Res 68:7711–7717. https://doi.org/10.1158/0008-5472.CAN-08-1949
Cheng J, Cashman JR (2020) Pancreatic cancer drug-sensitivity predicted by synergy of p53-Activator wnt Inhibitor-2 (PAWI-2) and protein biomarker expression. Investig New Drugs. https://doi.org/10.1007/s10637-020-00998-z
Cashman JR, Mercola M, Schade D, Tsuda M (2013) Compounds for inhibition of cancer cell proliferation. Google Patents. 2013:US 13/748,70
Okolotowicz KJ, Dwyer M, Ryan D, Cheng J, Cashman EA, Moore S et al (2018) Novel tertiary sulfonamides as potent anti-cancer agents. Bioorg Med Chem 26(15):4441–4451. https://doi.org/10.1016/j.bmc.2018.07.042
Cheng J, Dwyer M, Okolotowicz KJ, Mercola M, Cashman JR (2018) A novel inhibitor targets both wnt Signaling and ATM/p53 in Colorectal Cancer. Cancer Res 78(17):5072–5083. https://doi.org/10.1158/0008-5472.CAN-17-2642
Cheng J, Okolotowicz KJ, Ryan D, Mose E, Lowy AM, Cashman JR (2019) Inhibition of invasive pancreatic cancer: restoring cell apoptosis by activating mitochondrial p53. Am J Cancer Res 9(2):390–405
Cheng J, Moore S, Gomez-Galeno J, Lee DH, Okolotowicz KJ, Cashman JR (2019) A Novel Small Molecule inhibits Tumor Growth and synergizes effects of Enzalutamide on prostate Cancer. J Pharmacol Exp Ther 371(3):703–712. https://doi.org/10.1124/jpet.119.261040
Cheng J, Cashman JR (2020) PAWI-2 overcomes tumor stemness and drug resistance via cell cycle arrest in integrin β3-KRAS dependent pancreatic cancer stem cells. Sci Rep 10:9162–9173. https://doi.org/10.1038/s41598-020-65804-5
Cheng J, Cashman JR (2020) PAWI-2: a novel inhibitor for eradication of Cancer. Med Chem Res 29:1147–1159. https://doi.org/10.1007/s00044-020-02575-8
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 25:402–408
Tseng WW, Winer D, Kenkel JA, Choi O, Shain AH, Pollack JR, French R, Lowy AM, Engleman EG (2010) Development of an orthotopic model of invasive pancreatic cancer in an immunocompetent murine host. Clin Cancer Res 16:3684–3695
Lillie LE, Temple NJ, Florence LZ (1996) Reference values for young normal Sprague-Dawley rats: weight gain, hematology and clinical chemistry. Hum Exp Toxicol 15:612–616
Deer EL, Gonzalez-Hernandez J, Coursen JD, Shea JE, Ngatia J, Scaife CL, Firpo MA, Mulvihill SJ (2010) Phenotype and genotype of pancreatic cancer cell lines. Pancreas 39:425–435
Freed-Pastor WA, Prives C (2012) Mutant p53: one name, many proteins. Genes Dev 26:1268–1286
Xu J, Wang J, Hu Y, Qian J, Xu B, Chen H, Zou W, Fang JY (2014) Unequal prognostic potentials of p53 gain-of-function mutations in human cancers associate with drug-metabolizing activity. Cell Death Dis 5:e1108
Burris H, Storniolo AM (1997) Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer 33(Suppl 1):S18–22
Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, Tuveson DA (2012) Nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov 2:260–269
Acknowledgements
We thank Dr. David Cheresh of University of California, San Diego and The Scripps Research Institute for FGβ3 cells. We thank Drs. Ashutosh Tiwari and Deepak Rohila for help with the orthotopic xenograft surgeries. The contents of this publication are solely the responsibility of the author and do not necessarily represent the official view of The Conrad Prebys Foundation.
Funding
This work was supported by a grant award from The Conrad Prebys Foundation (Grant Number-44; J. R. Cashman) and by funds from the Human BioMolecular Research Institute. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of The Conrad Prebys Foundation.
Author information
Authors and Affiliations
Contributions
J.R.C. conceived the study conception and design. Material preparation was done by E.A.C. and J.R.C. conducted data collection and analysis. Both authors contributed to the study. The first draft of the manuscript was written by J.R.C and both authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Animal work was approved by the appropriate institutional committee. Animal work was conducted in accordance with the Guide for Care and Use of Laboratory Animals as adopted by the NIH. Formal approval was obtained from the IACUC of HBRI. The animal work did not require ethics approval.
Consent to participate
Not applicable. No human subjects were used.
Consent for publication
Not applicable. No human subjects were used.
Competing Interests
The authors do not have any competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cashman, J.R., Cashman, E.A. Effect of PAWI-2 on pancreatic cancer stem cell tumors. Invest New Drugs (2024). https://doi.org/10.1007/s10637-024-01447-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10637-024-01447-x