Summary
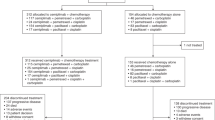
Background Dulanermin is a recombinant soluble human Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) that activates apoptotic pathways by binding to proapoptotic death receptor (DR) 4 and DR5. The purpose of this study was to evaluate the efficacy and safety of dulanermin combined with vinorelbine and cisplatin (NP) as the first-line treatment for patients with advanced non-small-cell lung cancer (NSCLC). Experimental design Patients were randomly assigned to receive NP chemotherapy (vinorelbine 25 mg/m2 on days 1 and 8 and cisplatin 30 mg/m2 on days 2 to 4) for up to six cycles plus dulanermin (75 μg/kg on days 1 to 14) or placebo every three weeks until disease progression, intolerable toxicity, or withdrawal of consent. The primary end point was progression-free survival (PFS), and the secondary end points included objective response rate (ORR), overall survival (OS), and safety evaluation. Results Between October 2009 and June 2012, 452 untreated patients with stage IIIB to IV NSCLC were randomly assigned to receive dulanermin plus NP (n = 342) and placebo plus NP (n = 110). Median PFS was 6.4 months in the dulanermin arm versus 3.5 months in the placebo arm (hazard ratio (HR), 0.4034; 95% CI, 0.3181 to 0.5117, p < 0.0001). ORR was 46.78% in the dulanermin arm versus 30.00% in the placebo arm (p = 0.0019). Median OS was 14.6 months in the dulanermin arm versus 13.9 months in the placebo arm (HR, 0.94; 95% CI, 0.74 to 1.21, p = 0.64). The most common grade ≥ 3 adverse events (AEs) were oligochromemia, leukopenia, neutropenia, and oligocythemia. Overall incidence of AEs, grade ≥ 3 AEs, and serious AEs were similar across the two arms. Conclusion Addition of dulanermin to the NP regimen significantly improved PFS and ORR. However, our results showed that the combination of dulanermin with chemotherapy had a synergic activity and favorable toxic profile in the treatment of patients with advanced NSCLC.



Similar content being viewed by others
References
American Cancer Society (2012) Non-small cell lung cancer 2012. American Cancer Society, Atlanta
American Cancer Society (2011) Cancer facts and figures 2011. American Cancer Society, Atlanta
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) (2011) Non-small cell lung cancer (version1.).
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A et al (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Johnson DH et al (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Ashkenazi A, Pai RC, Fong S, Leung S et al (1999) Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 104:155–162
Zhu L, Wang X, Li XM (2012) Mechanism of TRAIL induced tumor cell apoptosis and application of TRAIL based on tumor therapy. Acta Biophysica Sinica 28:448–456
Fan K, Luo L, Liu HH (2011) Research advance in TNF related apoptosis inducing ligand. J Chongqing Univ Technol 2:165–170
Johnstone RW, Ruefli AA, Lowe SW (2002) Apoptosis: a link between cancer genetics and chemotherapy. Cell 108:153–164
Schaefer U, Voloshanenko O, Willen D, Walczak H (2007) TRAIL: a multifunctional cytokine. Front Biosci 12:3813–3824
Camidge DR (2007) The potential of death receptor 4- and 5-directed therapies in the treatment of lung cancer. Clin Lung Cancer 8:413–419
Rowinsky EK (2005) Targeted induction of apoptosis in cancer management: the emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents. J Clin Oncol 23:9394–9407
Pollack IF, Erff M, Ashkenazi A (2001) Direct stimulation of apoptotic signaling by soluble Apo2l/tumor necrosis factor-related apoptosis-inducing ligand leads to selective killing of glioma cells. Clin Cancer Res 7:1362–1369
Ashkenazi A, Herbst RS (2008) To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest 118:1979–1990
Spierings DC, Vries EG, Timens W et al (2003) Expression of TRAIL and TRAIL death receptors in stage III non-small cell lung cancer tumors. Clin Cancer Res 9:3397–3405
Ashkenazi A, Holland P, Eckhardt SG (2008) Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/tumor necrosis factor-related apoptosis inducing ligand (rhApo2L/TRAIL). J Clin Oncol 26:3621–3630
Subbiah V, Brown RE, Buryanek J, Trent J et al (2012) Targeting the apoptotic pathway in chondrosarcoma using recombinant human Apo2L/TRAIL (Dulanermin), a dual Proapoptotic receptor (DR4/DR5) agonist. Mol Cancer Ther 11:2541–2546
Zhou SY, Chen SS, Liu P, Luo P, **ng PY et al (2007) Phase I clinical tolerance trial of recombinant human Apo-2 ligand in patients with advanced cancer. Chi J New Drugs 16:71–75
Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S et al (2010) Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol 28:2839–2846
Soria JC, Smit E, Khayat D et al (2010) Phase 1b study of dulanermin (recombinant human Apo2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 28:1527–1533
Soria JC, Mark Z, Zatloukal P, Szima B, Albert I et al (2011) Randomized phase II study of Dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non–small-cell lung cancer. J Clin Oncol 29:4442–4451
Acknowledgements
The authors thank the patients who participated in this study and express appreciation of the assistance and understanding of their families. The authors also thank the study-site staff for their support.
Funding
The work was also funded by Shanghai Biomedical Technology fund (grant number 08431901100), National Innovation Fund (grant number 10C26213100701), and National Science and Technology Major Project fund (grant number 2010ZX09401-301).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors received research funding from Gebaide Biotechnology Co., Ltd. for conducting the study and preparing this report. No potential conflicts of interest were disclosed.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual patients included in the study.
Rights and permissions
About this article
Cite this article
Ouyang, X., Shi, M., Jie, F. et al. Phase III study of dulanermin (recombinant human tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand) combined with vinorelbine and cisplatin in patients with advanced non-small-cell lung cancer. Invest New Drugs 36, 315–322 (2018). https://doi.org/10.1007/s10637-017-0536-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0536-y