Abstract
Introduction
Cytolethal distending toxin (Cdt) is one of the bacterial toxins that present in a variety of gram-negative human pathogens, such as E. coli, Salmonella spp., and Campylobacter spp. CDT is composed of three subunits encoded by three adjacent genes, including cdtA, cdtB, and cdtC. cdtB has been shown to have toxic activity and cause DNA damage in host cells. Despite its presence in different bacterial species, the role of CdtB in acute and chronic infections, such as gastroenteritis and irritable bowel syndrome (IBS), is unclear. To analyze this correlation, we studied the prevalence of cdtB among different enteropathogenic bacteria in patients with gastroenteritis and IBS compared with healthy people.
Materials and Methods
In this cross-sectional descriptive study, 230 stool samples were collected from patients with gastroenteritis, IBS, and healthy people. The presence of CdtB encoding bacteria, including Escherichia coli, Campylobacter spp., Yersinia entercolitica, Providencia alkalifacience, and Salmonella enterica, was examined by polymerase chain reaction using genus-specific primers.
Results
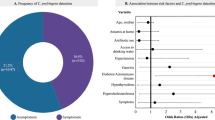
Out of 230 stool samples, CdtB encoding Campylobacter spp. were found in 34.6% (52/150), 6.25% (5/80), and 4% (2/50) of the patients with gastroenteritis, IBS, and the control group, respectively. Carriage of CdtB encoding Salmonella enterica was characterized among 5.3% (8/150) of the patients with gastroenteritis and 17.5% (14/80) of the IBS patients. Although none of the patients carried CdtB encoding E. coli and Providencia spp., cdtB of Y. enterocolitica was detected in one of the patients with gastroenteritis (0.6%). Statistical analysis showed significant correlation between infection with CdtB encoding Campylobacter spp. and IBS-D subtype. No significant correlation was found between infection with CdtB encoding bacteria and other clinical and demographic data.
Conclusion
Our results confirmed a relatively higher frequency of CdtB encoding bacteria in the intestine of patients with gastroenteritis and those with IBS compared with healthy individuals. Regarding the frequency of CdtB encoding Salmonella and Campylobacter bacteria, it was proposed that infection with these enteropathogens could be considered a risk factor for the development or progression of IBS among Iranian patients. Further studies are needed to establish this involvement.
Similar content being viewed by others
References
Ma LT, Galán JE. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect Immun. 2001;69:4358–4365.
Pons BJ, Vignard J, Mirey G. Cytolethal distending toxin subunit B: a review of structure–function relationship. Toxins. 2019;11:595.
Elwell CA, Dreyfus LA. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Molecular Microbiol. 2000;37:952–963.
Pickett CL, Pesci EC, Cottle DL, Russell G, Erdem AN, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter spp cdtB gene. Infect Immun. 1996;64:2070–2078.
Mezal EH, Bae D, Khan AA. Detection and functionality of the CdtB, PltA, and PltB from Salmonella enterica serovar Javiana. Pathogen Dis. 2014;72:95–103.
Osek J. Detection of cytolethal distending toxin genes in Shiga toxin-producing Escherichia coli isolated from different sources. Bull Vet Inst Pulawy. 2005;49:153–156.
Lluque A, Riveros M, Prada A, Ochoa TJ, Ruiz J. Virulence and antimicrobial resistance in Campylobacter spp from a Peruvian pediatric cohort. Scientifica. 2017;9:2017.
Humphries RM, Linscott AJ. Laboratory diagnosis of bacterial gastroenteritis. Clinical Microbiol. Rev. 2015;28:3–31.
Schwille-Kiuntke J, Enck P, Zendler C, Krieg M, Polster A, Klosterhalfen S, Autenrieth I, Zipfel S, Frick JS. Postinfectious irritable bowel syndrome: follow-up of a patient cohort of confirmed cases of bacterial infection with Salmonella or Campylobacter. Neurogastroenterology & Motil. 2011;23
Marshall JK, Thabane M, Borgaonkar MR, James C. Postinfectious irritable bowel syndrome after a food-borne outbreak of acute gastroenteritis attributed to a viral pathogen. Clin Gastroenterol Hepatol. 2007;5:457–460.
Thornley JP, Jenkins D, Neal K, Wright T, Brough J, Spiller RC. Relationship of Campylobacter toxigenicity in vitro to the development of postinfectious irritable bowel syndrome. J Infect Dis. 2001;184:606–609.
Shukla R, Ghoshal U, Dhole TN, Ghoshal UC. Fecal microbiota in patients with irritable bowel syndrome compared with healthy controls using real-time polymerase chain reaction: an evidence of dysbiosis. Digest Dis Sci. 2015;60:2953–2962.
Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microbial Ecol Health Dis. 2015;26:26191.
Ganji L, Alebouyeh M, Shirazi MH, Eshraghi SS, Mirshafiey A, Daryani NE, Zali MR. Dysbiosis of fecal microbiota and high frequency of Citrobacter, Klebsiella spp, and Actinomycetes in patients with irritable bowel syndrome and gastroenteritis. Gastroenterol Hepatol From Bed to Bench 2016;9:325.
Chassard C, Dapoigny M, Scott KP, Crouzet L, Del’homme C, Marquet P, Martin JC, Pickering G, Ardid D, Eschalier A. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharm Therapeut. 2012;35:828–838.
Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. The microbiome and irritable bowel syndrome–a review on the pathophysiology, current research and future therapy. Front Microbiol. 2019;10:1136.
Pimentel M, Morales W, Rezaie A, Marsh E, Lembo A, Mirocha J, Leffler DA, Marsh Z, Weitsman S, Chua KS. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLoS One 2015;10:e126438.
Buff E. A diagnostic blood test for irritable bowel syndrome: a utopia? Revue Med Suisse 2015;11:1628.
Pimentel M, Morales W, Pokkunuri V, Brikos C, Kim SM, Kim SE, Triantafyllou K, Weitsman S, Marsh Z, Marsh E. Autoimmunity links vinculin to the pathophysiology of chronic functional bowel changes following Campylobacter jejuni infection in a rat model. Digest Dis Sci. 2015;60:1195–1205.
Schmulson M, Balbuena R, de Law CC. Clinical experience with the use of anti-CdtB and anti-vinculin antibodies in patients with diarrhea in Mexico. Revista de Gastroenterología de México (English Edition) 2016;81:236–239.
Pimentel M, Chang C: Methods of comparing anti-vinculin and anti-cytolethal distending toxin antibodies as they relate to irritable bowel syndrome. In.: Google Patents; 2017.
Peters KE, Chang Y-C, Duhamel GE, Sultan AA, Doiphode S, Ibrahim EM, Mohammed HO. Cytolethal distending toxin in Salmonella and Campylobacter spp isolated from food animals and gastroenteritis cases in Qatar. Global J. Health Sci. 2017;9:34.
Burliaeva E, Bulakhov A, Pilipenko V, Isakov V, Sheveleva S. Campylobacter in stool of patients with irritable bowel syndrome. Eksperimental'naia i klinicheskaia gastroenterologiia= Exp & Clin Gastroenterol. 2010: 53–57.
Pokkunuri V, Pimentel M, Morales W, Jee S-R, Alpern J, Weitsman S, Marsh Z, Low K, Hwang L, Khoshini R. Role of cytolethal distending toxin in altered stool form and bowel phenotypes in a rat model of post-infectious irritable bowel syndrome. J Neurogastroenterol Motil. 2012;18:434.
Scuron MD, Boesze-Battaglia K, Dlakić M, Shenker BJ. The cytolethal distending toxin contributes to microbial virulence and disease pathogenesis by acting as a tri-perditious toxin. Front Cellular Infect Microbiol. 2016;6:168.
Morales W, Triantafyllou K, Parodi G, Weitsman S, Park SC, Rezaie A, Pichetshote N, Lin E, Pimentel M. Immunization with cytolethal distending toxin B produces autoantibodies to vinculin and small bowel bacterial changes in a rat model of postinfectious irritable bowel syndrome. Neurogastroenterol Motil : the Official J Eur Gastrointest Motil Soc. 2020;32:e13875.
Pons BJ, Vignard J, Mirey G. Cytolethal distending toxin subunit b: a review of structure-function relationship. Toxins (Basel) 2019;11:595.
Ganji L, Alebouyeh M, Shirazi MH, Zali MR. Comparative transcriptional analysis for Toll-like receptors, inflammatory cytokines, and apoptotic genes in response to different cytolethal-encoding and noncoding isolates of Salmonella enterica and Campylobacter jejuni from food and human stool. Microb Pathogen. 2019;133:103550.
Ng QX, Soh AYS, Loke W, Lim DY, Yeo WS. The role of inflammation in irritable bowel syndrome (IBS). J Inflamm Res. 2018;11:345–349.
Svendsen AT, Bytzer P, Engsbro AL. Systematic review with meta-analyses: does the pathogen matter in post-infectious irritable bowel syndrome? Scandinavian J Gastroenterol. 2019;54:546–562.
Pimentel M, Chatterjee S, Chang C, Low K, Song Y, Liu C, Morales W, Ali L, Lezcano S, Conklin J et al. A new rat model links two contemporary theories in irritable bowel syndrome. Dig Dis Sci 2008;53:982–989.
Miller R, Wiedmann M. Dynamic duo—the salmonella cytolethal distending toxin combines adp-ribosyltransferase and nuclease activities in a novel form of the cytolethal distending toxin. Toxins (Basel) 2016;8:121.
Haghjoo E, Galán JE. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proceed Natl Acad Sci. 2004;101:4614–4619.
Rodriguez-Rivera LD, Bowen BM, den Bakker HC, Duhamel GE, Wiedmann M. Characterization of the cytolethal distending toxin (typhoid toxin) in non-typhoidal Salmonella serovars. Gut Pathogen. 2015;7:19.
Williams K, Gokulan K, Shelman D, Akiyama T, Khan A, Khare S. Cytotoxic mechanism of cytolethal distending toxin in nontyphoidal Salmonella serovar (Salmonella Javiana) during macrophage infection. DNA and Cell Biol. 2015;34:113–124.
Cremon C, Stanghellini V, Pallotti F, Fogacci E, Bellacosa L, Morselli-Labate AM, Paccapelo A, Di Nardo G, Cogliandro RF, De Giorgio R et al. Salmonella gastroenteritis during childhood is a risk factor for irritable bowel syndrome in adulthood. Gastroenterology 2014;147:69–77.
Porter CK, Choi D, Cash B, Pimentel M, Murray J, May L, Riddle MS. Pathogen-specific risk of chronic gastrointestinal disorders following bacterial causes of foodborne illness. BMC Gastroenterol. 2013;13:46.
Meza-Segura M, Zaidi MB, Maldonado-Puga S, Huerta-Cantillo J, Chavez-Dueñas L, Navarro-Garcia F, Estrada-Garcia T: Cytolethal distending toxin-producing Escherichia coli strains causing severe diarrhoea in young Mexican children. JMM Case Rep. 2017, 4.
Pandey M, Khan A, Das SC, Sarkar B, Kahali S, Chakraborty S, Chattopadhyay S, Yamasaki S, Takeda Y, Nair GB. Association of cytolethal distending toxin locus cdtB with enteropathogenic Escherichia coli isolated from patients with acute diarrhea in Calcutta Indi. J Clin Microbiol. 2003;41:5277–5281.
Shima A, Hinenoya A, Asakura M, Sugimoto N, Tsukamoto T, Ito H, Nagita A, Faruque SM, Yamasaki S. Molecular characterizations of cytolethal distending toxin produced by Providencia alcalifaciens strains isolated from patients with diarrhea. Infect. Immun. 2012;80:1323–1332.
Acknowledgments
This study was part of a PhD thesis that was approved by the ethics committee of the Department of Pathobiology, Tehran University of Medical Sciences, Tehran, Iran (TUMS. ethics.code 92-02-27-22726). The authors thank all staff of Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, which helped us in this study. Indeed, many thanks go to Prof. Saeid Bouzari (Pasteure Institute of Iran, E. coli Reference Laboratory, Iran) for providing DNA of CdtB encoding E. coli.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing or potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ganji, L., Shirazi, M.H., Ebrahimi-Daryani, N. et al. Carriage of CdtB Encoding Campylobacter spp., Salmonella enterica, and Yersinia entercolitica in Patients with Gastroenteritis and Irritable Bowel Syndrome. Dig Dis Sci 67, 5522–5528 (2022). https://doi.org/10.1007/s10620-022-07468-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07468-x