Abstract
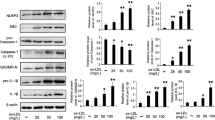
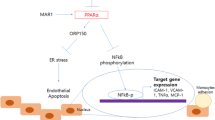
Atherosclerosis constitutes a proverbial pathogenic mechanism for cardio-cerebrovascular disease that accounts for the most common cause of disability and morbidity for human health worldwide. Endothelial dysfunction and inflammation are the key contributors to the progression of atherosclerosis. Glutaredoxin 2 (GLRX2) is abundantly existed in various tissues and possesses a range of pleiotropic efficacy including anti-oxidative and anti-inflammatory responses. However, its role in atherosclerosis is still undefined. Here, down-regulation of GLRX2 was validated in lipopolysaccha (LPS)-induced vascular endothelial cells (HUVECs). Moreover, elevation of GLRX2 reversed the inhibition of cell viability in LPS-treated HUVECs and decreased LPS-induced increases in cell apoptosis and caspase-3 activity. Additionally, enhancement of GLRX2 expression antagonized oxidative stress in HUVECs under LPS exposure by inhibiting ROS, lactate dehydrogenase and malondialdehyde production and increased activity of anti-oxidative stress superoxide dismutase. Notably, GLRX2 abrogated LPS-evoked transcripts and releases of pro-inflammatory cytokine (TNF-α, IL-6, and IL-1β), chemokine MCP-1 and adhesion molecule ICAM-1 expression. Furthermore, the activation of Nrf2/HO-1 signaling was demonstrated in LPS-stimulated HUVECs. Importantly, blockage of the Nrf2 pathway counteracted the protective roles of GLRX2 in LPS-triggered endothelial cell injury, oxidative stress and inflammatory response. Thus, these data reveal that GLRX2 may alleviate the progression of atherosclerosis by regulating vascular endothelial dysfunction and inflammation via the activation of the Nrf2 signaling, supporting a promising therapeutic approach for atherosclerosis and its complications.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Benjamin EJ, Blaha MJ, Chiuve SE et al (2017) Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135:e146–e603. https://doi.org/10.1161/CIR.0000000000000485
Bergstrom G, Persson M, Adiels M et al (2021) Prevalence of subclinical coronary artery atherosclerosis in the general population. Circulation 144:916–929. https://doi.org/10.1161/CIRCULATIONAHA.121.055340
Burns M, Rizvi SHM, Tsukahara Y et al (2020) Role of glutaredoxin-1 and glutathionylation in cardiovascular diseases. Int J Mol Sci 21:6803. https://doi.org/10.3390/ijms21186803
Chang X, Zhang T, Liu D et al (2021) Puerarin attenuates LPS-induced inflammatory responses and oxidative stress injury in human umbilical vein endothelial cells through mitochondrial quality control. Oxid Med Cell Longev 2021:6659240. https://doi.org/10.1155/2021/6659240
Chrysohoou C, Kollia N, Tousoulis D (2018) The link between depression and atherosclerosis through the pathways of inflammation and endothelium dysfunction. Maturitas 109:1–5. https://doi.org/10.1016/j.maturitas.2017.12.001
Diotte NM, **ong Y, Gao J, Chua BH, Ho YS (2009) Attenuation of doxorubicin-induced cardiac injury by mitochondrial glutaredoxin 2. Biochim Biophys Acta 1793:427–438. https://doi.org/10.1016/j.bbamcr.2008.10.014
Duan H, Zhang Q, Liu J, Li R, Wang D, Peng W, Wu C (2021) Suppression of apoptosis in vascular endothelial cell, the promising way for natural medicines to treat atherosclerosis. Pharmacol Res 168:105599. https://doi.org/10.1016/j.phrs.2021.105599
Fiorelli S, Porro B, Cosentino N et al (2019) Activation of Nrf2/HO-1 pathway and human atherosclerotic plaque vulnerability: an in vitro and in vivo study. Cells. https://doi.org/10.3390/cells8040356
Gimbrone MA Jr, Garcia-Cardena G (2016) Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118:620–636. https://doi.org/10.1161/CIRCRESAHA.115.306301
Hanschmann EM, Berndt C, Hecker C, Garn H, Bertrams W, Lillig CH, Hudemann C (2020) Glutaredoxin 2 reduces asthma-like acute airway inflammation in mice. Front Immunol 11:561724. https://doi.org/10.3389/fimmu.2020.561724
Hansson GK, Robertson AK, Soderberg-Naucler C (2006) Inflammation and atherosclerosis. Annu Rev Pathol 1:297–329. https://doi.org/10.1146/annurev.pathol.1.110304.100100
He Y, Yang G, Sun L et al (2021) SIRT6 inhibits inflammatory response through regulation of NRF2 in vascular endothelial cells. Int Immunopharmacol 99:107926. https://doi.org/10.1016/j.intimp.2021.107926
Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S (2016) Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res 118:535–546. https://doi.org/10.1161/CIRCRESAHA.115.307611
Horio E, Kadomatsu T, Miyata K et al (2014) Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler Thromb Vasc Biol 34:790–800. https://doi.org/10.1161/ATVBAHA.113.303116
Jian D, Wang Y, Jian L et al (2020) METTL14 aggravates endothelial inflammation and atherosclerosis by increasing FOXO1 N6-methyladeosine modifications. Theranostics 10:8939–8956. https://doi.org/10.7150/thno.45178
Lahera V, Goicoechea M, de Vinuesa SG, Miana M, de Heras N, Cachofeiro V, Luno J (2007) Endothelial dysfunction, oxidative stress and inflammation in atherosclerosis: beneficial effects of statins. Curr Med Chem 14:243–248. https://doi.org/10.2174/092986707779313381
Li C, **n H, Shi Y, Mu J (2021) Glutaredoxin 2 protects cardiomyocytes from hypoxia/reoxygenation-induced injury by suppressing apoptosis, oxidative stress, and inflammation via enhancing Nrf2 signaling. Int Immunopharmacol 94:107428. https://doi.org/10.1016/j.intimp.2021.107428
Li YY, Zhang GY, He JP, Zhang DD, Kong XX, Yuan HM, Chen FL (2017) Ufm1 inhibits LPS-induced endothelial cell inflammatory responses through the NF-kappaB signaling pathway. Int J Mol Med 39:1119–1126. https://doi.org/10.3892/ijmm.2017.2947
Liang S, Zhang J, Ning R et al (2020) The critical role of endothelial function in fine particulate matter-induced atherosclerosis. Part Fibre Toxicol 17:61. https://doi.org/10.1186/s12989-020-00391-x
Libby P (2021) The changing landscape of atherosclerosis. Nature 592:524–533. https://doi.org/10.1038/s41586-021-03392-8
Lu H, Fan Y, Qiao C et al (2017) TFEB inhibits endothelial cell inflammation and reduces atherosclerosis. Sci Signal 10:eaah4214. https://doi.org/10.1126/scisignal.aah4214
Meng Q, Pu L, Lu Q, Wang B, Li S, Liu B, Li F (2021) Morin hydrate inhibits atherosclerosis and LPS-induced endothelial cells inflammatory responses by modulating the NFkappaB signaling-mediated autophagy. Int Immunopharmacol 100:108096. https://doi.org/10.1016/j.intimp.2021.108096
Song P, Fang Z, Wang H et al (2020) Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health 8:e721–e729. https://doi.org/10.1016/S2214-109X(20)30117-0
Wen J, Li X, Zheng S, **ao Y (2020) Upregulation of glutaredoxin 2 alleviates oxygen-glucose deprivation/reoxygenation-induced apoptosis and ROS production in neurons by enhancing Nrf2 signaling via modulation of GSK-3beta. Brain Res 1745:146946. https://doi.org/10.1016/j.brainres.2020.146946
Wohua Z, Weiming X (2019) Glutaredoxin 2 (GRX2) deficiency exacerbates high fat diet (HFD)-induced insulin resistance, inflammation and mitochondrial dysfunction in brain injury: a mechanism involving GSK-3beta. Biomed Pharmacother 118:108940. https://doi.org/10.1016/j.biopha.2019.108940
Wu H, Lin L, Giblin F, Ho YS, Lou MF (2011) Glutaredoxin 2 knockout increases sensitivity to oxidative stress in mouse lens epithelial cells. Free Radic Biol Med 51:2108–2117. https://doi.org/10.1016/j.freeradbiomed.2011.09.011
Xu S, Ilyas I, Little PJ et al (2021) Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev 73:924–967. https://doi.org/10.1124/pharmrev.120.000096
Yao G, Qi J, Zhang Z et al (2019) Endothelial cell injury is involved in atherosclerosis and lupus symptoms in gld.apoE(-) (/) (-) mice. Int J Rheum Dis 22:488–496. https://doi.org/10.1111/1756-185X.13458
Zhang Q, Liu J, Duan H, Li R, Peng W, Wu C (2021) Activation of Nrf2/HO-1 signaling: an important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J Adv Res 34:43–63. https://doi.org/10.1016/j.jare.2021.06.023
Zhao Z, Wang X, Zhang R et al (2021) Melatonin attenuates smoking-induced atherosclerosis by activating the Nrf2 pathway via NLRP3 inflammasomes in endothelial cells. Aging 13:11363–11380. https://doi.org/10.18632/aging.202829
Acknowledgements
No.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
YL: Data curation; Investigation; Methodology; Resources; Software; Supervision; Validation; Visualization; Writing—original draft. JG: Formal analysis; Software; Data curation, Validation, review & editing. QW: Data curation; Investigation; Methodology; Formal analysis; Resources; Software; Validation; Writing—review & editing. NW: Data curation; Methodology; Investigation; Formal analysis; Validation; Resources. LZ: Data curation; Software; Methodology; Formal analysis; Validation. ZW: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Software; Validation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Gong, J., Wang, Q. et al. Activation of the Nrf2/HO-1 axis by glutaredoxin 2 overexpression antagonizes vascular endothelial cell oxidative injury and inflammation under LPS exposure. Cytotechnology 76, 167–178 (2024). https://doi.org/10.1007/s10616-023-00606-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-023-00606-x