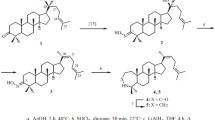
New derivatives with hydroxy, epoxy, hydroxyimino, acetoxy, lactol, methoxylactol, and indole groups in ring A and the side chain were synthesized via chemical transformations of dipterocarpol. The structure– cytotoxicity relationship was described for the dipterocarpol derivatives.


Similar content being viewed by others
References
T. Akihisa, H. Tokuda, M. Ukiya, T. Suzuki, F. Enjo, K. Koike, T. Nikado, and H. Nishino, Chem. Pharm. Bull., 52, 153 (2004).
F. Inada, M. Somekawa, and H. Murata, Chem. Pharm. Bull., 41, 617 (1993).
G. V. Platonov, A. D. Zorina, M. A. Gordon, N. P. Chizhov, L. V. Balykina, Yu. D. Mikhailov, D. R. Ivanen, T. K. Qui, and A. G. Shavva, Khim.-farm. Zh., 29, 42 (1995).
T. Akihisa, J. Ogihara, J. Kato, K. Yasukawa, M. Ukiya, S. Yamanouchi, and K. Oishi, Lipids, 36, 507 (2001).
D. Scholz, K. Baumann, M. Grassberger, B. Wolff-Winiski, G. Rihs, H. Walter, and J. G. Meingassner, Bioorg. Med. Chem. Lett., 14, 2983 (2004).
J.-M. Zhao, N. Li, C.-F. Wu, H.-R. Piao, and Y.-Q. Zhao, Bioorg. Med. Chem. Lett., 21, 1027 (2011).
N. H. Tung, G. Y. Song, J.-A. Kim, J.-H. Hyun, H.-K. Kang, and Y. H. Kim, Bioorg. Med. Chem. Lett., 20, 309 (2010).
J. Phongmaykin, T. Kumamoto, T. Ishikawa, E. Saifah, and R. Suttisri, Nat. Prod. Res., 25 (17), 1621 (2011).
M. Ukiya, T. Kikuchi, H. Tokuda, K. Tabata, Y. Kimura, T. Arai, Y. Ezaki, O. Oseto, T. Suzuki, and T. Akihisa, Chem. Biodiversity, 7, 1871 (2010).
D. Schmitz, J. Zapp, and R. Bernhardt, FEBS J., 279, 1663 (2012).
H. Hasegawa, J. Pharmacol. Sci., 95, 153 (2004).
M. C. Alley, D. A. Scudiero, P. A. Monks, M. L. Hursey, M. J. Czerwinski, D. L. Fine, B. J. Abbott, J. G. Mayo, R. H. Shoemaker, and M. R. Boyd, Cancer Res., 48 (3), 589 (1988).
M. R. Grever, S. A. Schepartz, and B. A. Chabner, Semin. Oncol., 19, 622 (1992).
M. R. Boyd and K. D. Paull, Drug. Dev., 34, 91 (1995).
R. H. Shoemaker, Nat. Rev. Cancer, 6, 813 (2006).
C. C. Lee and P. Houghton, J. Ethnopharmacol., 100, 237 (2005).
L. Q. Tran and K. Q. Tran, J. Chem., 36, 8 (1998).
A. D. Zorina, L. V. Balykina, O. V. Nazarova, and A. A. Rebezov, Zh. Prikl. Khim., 79, 663 (2006).
D. Sladowski, S. J. Steer, R. H. Chothier, and M. Balls, J. Immunol. Methods, 157, 203 (1993).
Acknowledgment
The work was supported financially by grants of the National Foundation for Science and Technology Development of Vietnam (Nafosted) and the RFBR No. 10-03-90303 and No. 11-03-12144. We thank the NCI for determining the in vitro antitumor activity of 1–4, 6, 7, and 12.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 1, January– February, 2013, pp. 51–57.
Rights and permissions
About this article
Cite this article
Huong, D.T.T., Thuy, T.T.T., Hien, T.T. et al. Synthesis and Cytotoxicity of Derivatives of Dipterocarpol, a Metabolite of Dipterocarpus alatus . Chem Nat Compd 49, 58–65 (2013). https://doi.org/10.1007/s10600-013-0505-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-013-0505-4