Abstract
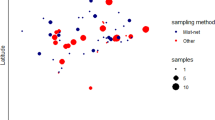
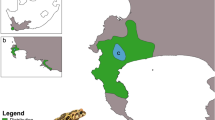
Wildlife species impacted by habitat loss and fragmentation often require conservation efforts to maintain populations. Long-nosed bandicoots (Perameles nasuta) still persist within the highly urbanised matrix of northern Sydney (Australia). One population at North Head, Sydney, is currently listed as an Endangered population due to its small size, apparent isolation and other threats. To support future management, we used 1,446 single nucleotide polymorphism markers (SNPs) from 167 bandicoots to: i) assess the assumption of isolation and determine if genetic structuring is present between North Head and individuals from 11 other localities in northern Sydney, and ii) investigate genetic diversity over time in the North Head population from 2002 to 2018. Analyses confirmed population structuring and genetic divergence between North Head and greater northern Sydney. Three distinct populations were identified that corresponded to geographic localities (North Head, northern Sydney and Mosman). All populations were significantly differentiated (FST = 0.171–0.345), suggesting local genetic drift between localities. North Head genetic diversity indices estimated between 2002 to 2018 showed relatively constant levels of allelic richness (1.90–2.00) and observed heterozygosity (HO = 0.231–0.310) along with minor levels of inbreeding (FIS 0.020–0.052). The identification of some individuals sampled on North Head that were assigned to other populations suggests some sporadic geneflow into the population has occurred and may have assisted with maintaining genetic diversity. These data suggest that the North Head population is distinct from other northern Sydney populations and has relatively constant levels of genetic diversity.





Similar content being viewed by others
Availability of data and materials
Genomic data has been uploaded to DRYAD. Accession number: https://doi.org/10.5061/dryad.f7m0cfxvk.
References
Anson J (2015) Long-nosed bandicoot (Perameles nasuta) monitoring, North Head Sanctuary. Manly, Australian Wildlife Conservancy, Sydney
Allendorf FW 1983. Isolation, gene flow and genetic differentiation among populations. Genetics and Conservation: A Reference for Managing Wild Animal and Plant Populations. (eds. C.M., Schonewald-Cox, S.M., Chambers, B., MacBryde, W.L., Thomas), 51–65. Menlo Park, California.
Backus VL, Bryant EH, Hughes CR, Meffert LM (1995) Effect of migration or inbreeding followed by selection on low-founder-number populations: Implications for captive breeding programs. Conserv Biol 9:1216–1224
Banks PB 2004 Population viability analysis in urban wildlife management: Modelling management options for Sydney’s quarantined bandicoots. Urban Wildlife: More Than Meets the Eye. (eds. D., Lunney and S., Burgin), 70–77. Royal Zoological Society of New South Wales: Sydney, Australia.
Chambers LK, Dickman CR (2002) Habitat selection of the long-nosed bandicoot, Perameles nasuta (mammalia, peramelidae), in a patchy urban environment. Austral Ecol 27:334–342
Dennison S, Frankham G, Neaves L, Flanagan C, FitzGibbon S, Eldridge M, Johnson R (2017) Population genetics of the koala (Phascolarctos cinereus) in north-eastern New South Wales and south-eastern Queensland. Aust J Zool 64:402–412
Dexter N, Hudson M, Carter T, Macgregor C (2011) Habitat-dependent population regulation in an irrupting population of long-nosed bandicoots (Perameles nasuta). Austral Ecol 36:745–754
Dowle M (2012) A comparison of two species of bandicoots (Perameles nasuta & Isoodon obesulus) influenced by urbanisation: Population characteristics, genetic diversity, public perceptions, stress and parasites. Macquarie University, Sydney, New South Wales
Dowle M, Deane EM (2009) Attitudes to native bandicoots in an urban environment. Eur J Wildl Res 55:45–52
Dowle M, Webster KN, Deane E (2013) Faecal glucocorticoid metabolite concentrations in the free-ranging bandicoots (Perameles nasuta and Isoodon obesulus) of northern Sydney. Australian Mammalogy 35:1–7
Earl DA, Von Holdt B (2012) Structure Harvester: A website and program for visualizing structure output and implementing the evanno method. Conserv Genet Resour 4:359–361
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol Ecol 14:2611–2620
Ewart KM, Johnson RN, Ogden R, Joseph L, Frankham GJ, Lo N (2019) Museum specimens provide reliable SNP data for population genomic analysis of a widely distributed but threatened cockatoo species. Mol Ecol Resour 19:1381–1688
Fischer J, Lindenmayer DB (2007) Landscape modification and habitat fragmentation: A synthesis. Glob Ecol Biogeogr 16:265–280
Frankham R (1997) Do island populations have less genetic variation than mainland populations? Heredity 78:311–327
Frankham R (2010) Where are we in conservation genetics and where do we need to go? Conserv Genet 11:661–663
Frankham R (2015) Genetic rescue of small inbred populations: Meta-analysis reveals large and consistent benefits of gene flow. Mol Ecol 24:2610–2618
Frankham GJ, Handasyde KA, Norton M, Murray A, Eldridge MDB (2014) Molecular detection of intra-population structure in a threatened potoroid, Potorous tridactylus: Conservation management and sampling implications. Conserv Genet 15:547–560
Frankham R, Ballou JD, Ralls K, Eldridge MDB, Dudash MR, Fenster C, Lacy RC and Sunnucks P (2017) Genetic management of fragmented animal and plant populations. Oxford University Press
Fuentes-Pardo AP, Ruzzante DE (2017) Whole-genome sequencing approaches for conservation biology: Advantages, limitations and practical recommendations. Mol Ecol 26:5369–5406
Goossens B, Sharma R, Othman N, Kun-Rodrigues C, Sakong R, Ancrenaz M, Ambu LN, Jue NK, O’Neill RJ, Bruford MW (2016) Habitat fragmentation and genetic diversity in natural populations of the Bornean elephant: Implications for conservation. Biol Cons 196:80–92
Goudet J (2005) HIERFSTAT, a package for R to compute and test hierarchical F-statistics. Mol Ecol Notes 5:184–186
GruberUnmack PJ B, Berry OF, Georges A (2018) DARTR: An r package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Mol Ecol Resour 18:691–699
Janes JK, Miller JM, Dupuis JR, Malenfant RM, Gorrell JC, Cullingham CI, Andrew RL (2017) The K = 2 conundrum. Mol Ecol 26:3594–3602
Jombart T (2008) ADEGENET: A r package for the multivariate analysis of genetic markers. Bioinformatics 24:1403–1405
Jombart T, Devillard S, Balloux F (2010) Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet 11:94
Keenan K, McGinnity P, Cross TF, Crozier WW, Prodöhl PA (2013) DIVERSITY An r package for the estimation and exploration of population genetics parameters and their associated errors. Methods Ecol Evol 4:782–788
Keyghobadi N (2007) The genetic implications of habitat fragmentation for animals. Can J Zool 85:1049–1064
Kilian A, Wenzl P, Huttner E, Carling J, **a L, Blois H, Uszynski G (2012) Diversity arrays technology: A generic genome profiling technology on open platforms. Methods Mol Biol 888:67–89
Kopelman NM, Mayzel J, Jakobsson M, Rosenberg NA, Mayrose I (2015) CLUMPAK: A program for identifying clustering modes and packaging population structure inferences across K. Mol Ecol Resour 15:1179–1191
Lacy RC (1987) Loss of genetic diversity from managed populations: Interacting effects of drift, mutation, immigration, selection, and population subdivision. Conserv Biol 1:143–158
Lancaster ML, Taylor AC, Cooper SJ, Carthew SM (2011) Limited ecological connectivity of an arboreal marsupial across a forest/plantation landscape despite apparent resilience to fragmentation. Mol Ecol 20:2258–2271
Lande R, Barrowclough GF, 1987. Effective population size, genetic variation, and their use in population management: Viable Populations for Conservation. (eds. M.E., Soule), 87–123. Cambridge University Press, Cambridge, England.
Lott M, Wright B, Kemp L, Johnson R, Hogg C (2020) Genetic management of captive and reintroduced bilby populations. J Wildl Manag 84:20–32
Luikart G, England PR, Tallmon D, Jordan S, Taberlet P (2003) The power and promise of population genomics: From genoty** to genome ty**. Nat Rev Genet 4:981–994
Meirmans PG (2015) Seven common mistakes in population genetics and how to avoid them. Mol Ecol 24:3223–3231
Melville J, Haines ML, Boysen K, Hodkinson L, Kilian A, Smith Date KL, Potvin DA, Parris KM (2017) Identifying hybridization and admixture using SNPs: Application of the DArTseq platform in phylogeographic research on vertebrates. Royal Society Open Science 4:161061
Menges ES (1991) The application of minimum viable population theory to plants. Genetics Conservation Rare Plants 45:158–164
Moyle D, Hume I, Hill D (1995) Digestive performance and selective digesta retention in the long-nosed bandicoot, Perameles nasuta, a small omnivorous marsupial. J Comp Physiol B 164:552–560
New South Wales, Department of Primary Industries, 2020. Drought in NSW https://www.dpi.nsw.gov.au/climate-and-emergencies/droughthub/drought-in-nsw
New South Wales, Threatened Species Scientific Committee, 1997. Long-nosed bandicoot (Perameles nasuta) population, North Head - endangered population listing https://www.environment.nsw.gov.au/topics/animals-and-plants/threatened-species/nsw-threatened-species-scientific-committee/determinations/final-determinations/1996-1999/long-nosed-bandicoot-population-north-head-endangered-population-listing
Novembre J, Stephens M (2008) Interpreting principal component analyses of spatial population genetic variation. Nat Genet 40:646
Paradis E (2010) PEGAS: An r package for population genetics with an integrated–modular approach. Bioinformatics 26:419–420
Pembleton LW, Cogan NO, Forster JW (2013) STAMPP: An r package for calculation of genetic differentiation and structure of mixed-ploidy level populations. Mol Ecol Resour 13:946–952
Piggott M, Banks SC, MacGregor C, Lindenmayer DB (2018) Population genetic patterns in an irruptive species, the long-nosed bandicoot (Perameles nasuta). Conserv Genet 19:655–663
Piggott M, Banks SC, Taylor AC (2006) Population structure of brush-tailed rock-wallaby (Petrogale penicillata) colonies inferred from analysis of faecal DNA. Mol Ecol 15:93–105
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
RStudio Team, 2015. RStudio: Integrated Development for R. RStudio, Inc. Boston, MA. http://www.rstudio.com/.
Ralls K, Ballou JD, Dudash MR, Eldridge MDB, Fenster CB, Lacy RC, Sunnucks P, Frankham R (2018) Call for a paradigm shift in the genetic management of fragmented populations. Conserv Lett 11:e12412
Rosenberg NA (2004) DISTRUCT: A program for the graphical display of population structure. Mol Ecol Notes 4:137–138
Saccheri I, Kuussaari M, Kankare M, Vikman P, Fortelius W, Hanski I (1998) Inbreeding and extinction in a butterfly metapopulation. Nature 392:491–494
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: A review. Conserv Biol 5:18–32
Scott L, Hume., I Dickman., C., (1999) Ecology and population biology of long-nosed bandicoots (Perameles nasuta) at North Head, Sydney Harbour National Park. Wildl Res 26:805–821
Short J (2016) Predation by feral cats key to the failure of a long-term reintroduction of the western barred bandicoot (Perameles bougainville). Wildl Res 43:38–50
Spielman D, Brook BW, Briscoe DA, Frankham R (2004) Does inbreeding and loss of genetic diversity decrease disease resistance? Conserv Genet 5:439–448
Sunnucks P, Hales DF (1996) Numerous transposed sequences of mitochondrial cytochrome oxidase i–ii in aphids of the genus Sitobion (Hemiptera: Aphididae). Mol Biol Evol 13:510–524
Tea YK, Van Der Wal C, Ludt WB, Gill AC, Lo N, Ho SY (2019) Boomeranging around Australia: Historical biogeography and population genomics of the anti-equatorial fish Microcanthus strigatus (Teleostei: Microcanthidae). Mol Ecol 28:3771–3785
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Winnard AL, Coulson G (2008) Sixteen years of eastern barred bandicoot Perameles gunnii reintroductions in Victoria: A review. Pac Conserv Biol 14:34–53
Acknowledgements
Thanks to Australian Wildlife Conservancy, NSW National Parks and Wildlife Service, Taronga Conservation Society and the Mammal Collection, Australian Museum for providing samples. Thanks to the Sydney Harbour Federation Trust for funding. Thanks also to M. Hall, E. Lee, L. de Gail, and J. Dargan for long term sample collection, the Australian Centre for Wildlife Genomics (Australian Museum) staff for assistance with laboratory work, and C. Van Der Wal, K. Yi Tea, M. Lott and K. Ewart for assistance with data analysis. Funding for this work was provided by the Sydney Harbour Federation Trust and Australian Wildlife Conservancy.
Funding
This study was funded by the Sydney Harbour Federation Trust and Australian Wildlife Conservancy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The methods were performed in accordance with the following ethics approvals and licensing. Animal Research Authority (ARA) – Secretary’s Animal Care and Ethics Committee approval for Biodiversity Conservation Restoration, North Head. Department of Primary Industry (DPI) Scientific Licence 101,333—Biodiversity conservation and restoration at North Head. Office of Environment and Heritage (OEH) Animal Research Authority (ARA) – Secretary’s Animal Care and Ethics Committee approval for Long-nosed Bandicoot Recovery Program AEC 000,214/05 (OEH).
Consent for publication
All authors have reviewed the manuscript and have provided consent to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Nelson, H.V., Frankham, G.J., Leo, V. et al. Conservation genomics of the ‘Endangered’ long-nosed bandicoot (Perameles nasuta) population at North Head, Sydney, Australia. Conserv Genet 22, 745–756 (2021). https://doi.org/10.1007/s10592-021-01356-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-021-01356-z