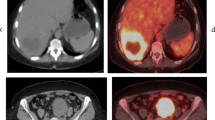
Abstract
Approximately 25% of those who are diagnosed with colorectal cancer will develop colorectal liver metastases (CRLM) as their illness advances. Despite major improvements in both diagnostic and treatment methods, the prognosis for patients with CRLM is still poor, with low survival rates. Accurate employment of imaging methods is critical in identifying the most effective treatment approach for CRLM. Different imaging modalities are used to evaluate CRLM, including positron emission tomography (PET)/computed tomography (CT). Among the PET radiotracers, fluoro-18-deoxyglucose (18F-FDG), a glucose analog, is commonly used as the primary radiotracer in assessment of CRLM. As the importance of 18F-FDG-PET/CT continues to grow in assessment of CRLM, develo** a comprehensive understanding of this subject becomes imperative for healthcare professionals from diverse disciplines. The primary aim of this article is to offer a simplified and comprehensive explanation of PET/CT in the evaluation of CRLM, with a deliberate effort to minimize the use of technical nuclear medicine terminology. This approach intends to provide various healthcare professionals and researchers with a thorough understanding of the subject matter.













Similar content being viewed by others
References
Martin J et al (2020) Colorectal liver metastases: current management and future perspectives. World J Clin Oncol 11(10):761–808
Zhou H et al (2022) Colorectal liver metastasis: molecular mechanism and interventional therapy. Signal Transduct Target Ther 7(1):70
Akgül Ö et al (2014) Role of surgery in colorectal cancer liver metastases. World J Gastroenterol 20(20):6113
Zirakchian Zadeh M et al (2022) Real-time split-dose PET/CT-guided ablation improves colorectal liver metastasis detection and ablation zone margin assessments without the need for repeated contrast injection. Cancers (Basel) 14(24):6253
Bandar A, Hussain M, Kim NK (2017) Current status and future perspectives on treatment of liver metastasis in colorectal cancer. Oncol Rep 37(5):2553–2564
Takahashi H, Berber E (2020) Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg Nutr 9(1):49
Schirrmeister H, Arslandemir C (2010) Chapter 24—diagnosis of skeletal metastases in malignant extraskeletal cancers. In: Heymann D (ed) Bone cancer. Academic Press, San Diego, pp 283–294
Hofman MS, Hicks RJ (2016) How we read oncologic FDG PET/CT. Cancer Imaging 16(1):35
Cipe G et al (2013) Routine use of positron-emission tomography/computed tomography for staging of primary colorectal cancer: does it affect clinical management? World J Surg Oncol 11(1):1–8
Deng J, Tang J, Shen N (2014) Meta-analysis of diagnosis of liver metastatic cancers: comparison of 18 FDG PET-CT and gadolinium-enhanced MRI. J Med Imaging Radiat Oncol 58(5):532–537
Rohren EM et al (2002) The role of F-18 FDG positron emission tomography in preoperative assessment of the liver in patients being considered for curative resection of hepatic metastases from colorectal cancer. Clin Nucl Med 27(8):550–555
Ruers T et al (2002) Value of positron emission tomography with [F-18] fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol 20(2):388–395
Sahani DV et al (2005) Detection of liver metastases from adenocarcinoma of the colon and pancreas: comparison of mangafodipir trisodium–enhanced liver MRI and whole-body FDG PET. Am J Roentgenol 185(1):239–246
D’souza MM et al (2009) Prospective evaluation of CECT and 18F-FDG-PET/CT in detection of hepatic metastases. Nucl Med Commun 30(2):117–125
Tahtabasi M, Erturk SM, Basak M (2021) Comparison of MRI and 18F-FDG PET/CT in the liver metastases of gastrointestinal and pancreaticobiliary tumors. Sisli Etfal Hastan Tip Bul 55(1):12–17
Yang M et al (2003) Comparison of MR and PET imaging for the evaluation of liver metastases. J Magn Reson Imaging 17(3):343–349
Beiderwellen K et al (2015) Accuracy of [18F]FDG PET/MRI for the detection of liver metastases. PLoS ONE 10(9):e0137285
Sivesgaard K et al (2018) Diagnostic accuracy of CE-CT, MRI and FDG PET/CT for detecting colorectal cancer liver metastases in patients considered eligible for hepatic resection and/or local ablation. Eur Radiol 28(11):4735–4747
Tsili AC et al (2020) Imaging of colorectal cancer liver metastases using contrast-enhanced US, multidetector CT, MRI, and FDG PET/CT: a meta-analysis. Acta Radiol 62(3):302–312
Zirakchian Zadeh M et al (2020) Correlation of whole-bone marrow dual-time-point (18)F-FDG, as measured by a CT-based method of PET/CT quantification, with response to treatment in newly diagnosed multiple myeloma patients. Am J Nucl Med Mol Imaging 10(5):257–264
Dirisamer A et al (2008) Dual-time-point FDG-PET/CT for the detection of hepatic metastases. Mol Imaging Biol 10(6):335–340
de Geus-Oei LF et al (2006) FDG-PET in colorectal cancer. Cancer Imaging 6(Special Issue A):S71-81
Staib L et al (2000) Is 18F-fluorodeoxyglucose positron emission tomography in recurrent colorectal cancer a contribution to surgical decision making? Am J Surg 180(1):1–5
Imdahl A et al (2000) Impact of 18 F-FDG-positron emission tomography for decision making in colorectal cancer recurrences. Langenbecks Arch Surg 385:129–134
Kalff V et al (2002) The clinical impact of 18F-FDG PET in patients with suspected or confirmed recurrence of colorectal cancer: a prospective study. J Nucl Med 43(4):492–499
Huebner RH et al (2000) A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med 41(7):1177–1189
Wiering B et al (2005) The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer 104(12):2658–2670
Zhou N et al (2021) The Value of (18)F-FDG PET/CT and abdominal PET/MRI as a one-stop protocol in patients with potentially resectable colorectal liver metastases. Front Oncol 11:714948
Grassetto G et al (2010) Additional value of FDG-PET/CT in management of “solitary” liver metastases: preliminary results of a prospective multicenter study. Mol Imag Biol 12:139–144
Zirakchian Zadeh M et al (2022) A review of different methods used for quantification and assessment of FDG-PET/CT in multiple myeloma. Nucl Med Commun 43(4):378–391
Im HJ et al (2018) Current methods to define metabolic tumor volume in positron emission tomography: which one is better? Nucl Med Mol Imaging 52(1):5–15
**a Q et al (2015) Prognostic significance of 18FDG PET/CT in colorectal cancer patients with liver metastases: a meta-analysis. Cancer Imaging 15(1):19
de Geus-Oei LF et al (2006) FDG-PET for prediction of survival of patients with metastatic colorectal carcinoma. Ann Oncol 17(11):1650–1655
Zwezerijnen GJC et al (2023) Reproducibility of [18F]FDG PET/CT liver SUV as reference or normalisation factor. Eur J Nucl Med Mol Imaging 50(2):486–493
Grut H et al (2022) Metabolic tumor volume predicts long-term survival after transplantation for unresectable colorectal liver metastases: 15 years of experience from the SECA study. Ann Nucl Med 36(12):1073–1081
Zalom M et al (2012) FDG PET/CT as a prognostic test After 90Y radioembolization in patients with metastatic hepatic disease. Clin Nucl Med 37(9):862
Higashi K et al (2002) 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med 43(1):39–45
Vansteenkiste JF et al (1999) Prognostic importance of the standardized uptake value on 18F-fluoro-2-deoxy-glucose–positron emission tomography scan in non–small-cell lung cancer: an analysis of 125 cases. J Clin Oncol 17(10):3201–3206
Bijlstra OD et al (2022) The value of (18)F-FDG-PET-CT imaging in treatment evaluation of colorectal liver metastases: a systematic review. Diagnostics (Basel) 12(3):715
Burger IA et al (2013) Correlation between therapy response assessment using FDG PET/CT and histopathologic tumor regression grade in hepatic metastasis of colorectal carcinoma after neoadjuvant therapy. Ann Nucl Med 27:177–183
Lubezky N et al (2007) The role and limitations of 18-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scan and computerized tomography (CT) in restaging patients with hepatic colorectal metastases following neoadjuvant chemotherapy: comparison with operative and pathological findings. J Gastrointest Surg 11:472–478
Vicente AMG et al (2013) Response assessment of colorectal liver metastases with contrast enhanced CT/18F-FDG PET. Eur J Radiol 82(6):e255–e261
Tan MC et al (2007) Chemotherapy-induced normalization of FDG uptake by colorectal liver metastases does not usually indicate complete pathologic response. J Gastrointest Surg 11:1112–1119
Bacigalupo L et al (2010) Assessment of liver metastases from colorectal adenocarcinoma following chemotherapy: SPIO-MRI versus FDG-PET/CT. Radiol Med (Torino) 115(7):1087–1100
Lastoria S et al (2013) Early PET/CT scan is more effective than RECIST in predicting outcome of patients with liver metastases from colorectal cancer treated with preoperative chemotherapy plus bevacizumab. J Nucl Med 54(12):2062–2069
De Bruyne S et al (2012) Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br J Cancer 106(12):1926–1933
Mertens J et al (2013) Standardized added metabolic activity (SAM) IN 18F-FDG PET assessment of treatment response in colorectal liver metastases. Eur J Nucl Med Mol Imaging 40(8):1214–1222
Nishioka Y et al (2018) Fluorine-18-fluorodeoxyglucose positron emission tomography as an objective substitute for CT morphologic response criteria in patients undergoing chemotherapy for colorectal liver metastases. Abdom Radiol 43(5):1152–1158
Skougaard K et al (2014) CT versus FDG-PET/CT response evaluation in patients with metastatic colorectal cancer treated with irinotecan and cetuximab. Cancer Med 3(5):1294–1301
Heijmen L et al (2015) Multimodality imaging to predict response to systemic treatment in patients with advanced colorectal cancer. PLoS ONE 10(4):e0120823
Chiu KWH et al (2018) Long-term outcomes and recurrence pattern of 18F-FDG PET-CT complete metabolic response in the first-line treatment of metastatic colorectal cancer: a lesion-based and patient-based analysis. BMC Cancer 18(1):776
Kim DH et al (2012) Intermodality comparison between 3D perfusion CT and 18F-FDG PET/CT imaging for predicting early tumor response in patients with liver metastasis after chemotherapy: preliminary results of a prospective study. Eur J Radiol 81(11):3542–3550
Nemeth Z et al (2020) Metabolic parameters as predictors for progression free and overall survival of patients with metastatic colorectal cancer. Pathol Oncol Res 26(4):2683–2691
Correa-Gallego C et al (2015) Prospective evaluation of 18F-fluorodeoxyglucose positron emission tomography in patients receiving hepatic arterial and systemic chemotherapy for unresectable colorectal liver metastases. HPB (Oxford) 17(7):644–650
Sacks A et al (2011) Value of PET/CT in the management of liver metastases, part 1. Am J Roentgenol 197(2):W256–W259
Fernandez FG et al (2004) Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg 240(3):438
Ruers TJ et al (2009) Improved selection of patients for hepatic surgery of colorectal liver metastases with 18F-FDG PET: a randomized study. J Nucl Med 50(7):1036–1041
Oh JW et al (2016) Does the gadoxetic acid-enhanced liver MRI Impact on the treatment of patients with colorectal cancer? Comparison study with 18F-FDG PET/CT. Biomed Res Int 2016:8412071
Van Cutsem E et al (2016) Imaging in colorectal cancer: progress and challenges for the clinicians. Cancers 8(9):81
Lin Y-M et al (2021) Image-guided ablation for colorectal liver metastasis: principles, current evidence, and the path forward. Cancers 13(16):3926
Viganò L et al (2017) Positron emission tomography-computed tomography for patients with recurrent colorectal liver metastases: impact on restaging and treatment planning. Ann Surg Oncol 24:1029–1036
Petre EN, Sofocleous C (2017) Thermal ablation in the management of colorectal cancer patients with oligometastatic liver disease. Visc Med 33(1):62–68
Solbiati L et al (2012) Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 265(3):958–968
Shady W et al (2016) Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology 278(2):601–611
Veit P et al (2006) Detection of residual tumor after radiofrequency ablation of liver metastasis with dual-modality PET/CT: initial results. Eur Radiol 16:80–87
Sahin DA et al (2012) The utility of PET/CT in the management of patients with colorectal liver metastases undergoing laparascopic radiofrequency thermal ablation. Ann Surg Oncol 19:850–855
Cornelis F et al (2016) 18F-FDG PET/CT is an immediate imaging biomarker of treatment success after liver metastasis ablation. J Nucl Med 57(7):1052–1057
Nielsen K et al (2013) PET-CT after radiofrequency ablation of colorectal liver metastases: suggestions for timing and image interpretation. Eur J Radiol 82(12):2169–2175
Kuehl H et al (2008) Comparison of FDG-PET, PET/CT and MRI for follow-up of colorectal liver metastases treated with radiofrequency ablation: initial results. Eur J Radiol 67(2):362–371
Liu Z-y et al (2010) Early PET/CT after radiofrequency ablation in colorectal cancer liver metastases: is it useful? Chin Med J 123(13):1690–1694
Zirakchian Zadeh M et al (2023) Gradient-based volumetric PET parameters on immediate pre-ablation FDG-PET predict local tumor progression in patients with colorectal liver metastasis treated by microwave ablation. CardioVasc Int Radiol. https://doi.org/10.1007/s00270-023-03470-6
Cornelis FH et al (2018) Immediate postablation 18F-FDG injection and corresponding SUV are surrogate biomarkers of local tumor progression after thermal ablation of colorectal carcinoma liver metastases. J Nucl Med 59(9):1360–1365
Seraj SM et al (2019) Pretreatment volumetric parameters of FDG-PET predict the survival after Yttrium-90 radio-embolization in metastatic liver disease. Am J Nucl Med Mol Imaging 9(5):248–254
Shady W et al (2016) Metabolic tumor volume and total lesion glycolysis on FDG-PET/CT can predict overall survival after 90Y radioembolization of colorectal liver metastases: a comparison with SUVmax, SUVpeak, and RECIST 1.0. Eur J Radiol 85(6):1224–1231
Fendler WP et al (2013) Validation of several SUV-based parameters derived from 18F-FDG PET for prediction of survival after SIRT of hepatic metastases from colorectal cancer. J Nucl Med 54(8):1202–1208
Soydal C et al (2013) The prognostic value of quantitative parameters of 18F-FDG PET/CT in the evaluation of response to internal radiation therapy with yttrium-90 in patients with liver metastases of colorectal cancer. Nucl Med Commun 34(5):501–506
Zerizer I et al (2012) The role of early 18 F-FDG PET/CT in prediction of progression-free survival after 90 Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging 39:1391–1399
Shady W et al (2016) Surrogate imaging biomarkers of response of colorectal liver metastases after salvage radioembolization using 90Y-loaded resin microspheres. AJR Am J Roentgenolo 207(3):661
Sabet A et al (2015) Early post-treatment FDG PET predicts survival after 90 Y microsphere radioembolization in liver-dominant metastatic colorectal cancer. Eur J Nucl Med Mol Imaging 42:370–376
Jongen JM et al (2018) Anatomic versus metabolic tumor response assessment after radioembolization treatment. J Vasc Interventional Radiol 29(2):244–532
Sager S et al (2019) Comparison of PERCIST and RECIST criteria for evaluation of therapy response after yttrium-90 microsphere therapy in patients with hepatocellular carcinoma and those with metastatic colorectal carcinoma. Nucl Med Commun 40(5):461–468
Naydenov N et al (2023) 90Y activity quantification in PET/CT-guided biopsy specimens from colorectal hepatic metastases immediately after trans-arterial radioembolization using micro-CT and autoradiography. J Vasc Interventional Radiol. https://doi.org/10.1016/j.jvir.2023.05.022
Liu F-Y et al (2005) Utility of 2-fluoro-2-deoxy-D-glucose positron emission tomography in managing patients of colorectal cancer with unexplained carcinoembryonic antigen elevation at different levels. Dis Colon Rectum 48:1900–1912
Tutt A et al (2004) The role of positron emission tomography in the management of colorectal cancer. Colorectal Dis 6(1):2–9
Bast RC Jr et al (2001) 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 19(6):1865–1878
Pfister DG, Benson AB III, Somerfield MR (2004) Surveillance strategies after curative treatment of colorectal cancer. New Engl J Med 350(23):2375–2382
Rosen M et al (1998) Follow-up of colorectal cancer: a meta-analysis. Dis Colon Rectum 41:1116–1126
Renehan AG et al (2002) Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ 324(7341):813
Flamen P et al (1999) Additional value of whole-body positron emission tomography with fluorine-18–2-fluoro-2-deoxy-D-glucose in recurrent colorectal cancer. J Clin Oncol 17(3):894
Flanagan FL et al (1998) Utility of FDG-PET for investigating unexplained plasma CEA elevation in patients with colorectal cancer. Ann Surg 227(3):319
Maldonado A et al (2000) 16. FDG-PET in the detection of recurrence in colorectal cancer based on rising CEA level. Experience in 72 Patients. Clin Positron Imaging 3(4):170
Flamen P et al (2001) Unexplained rising carcinoembryonic antigen (CEA) in the postoperative surveillance of colorectal cancer: the utility of positron emission tomography (PET). Eur J Cancer 37(7):862–869
Libutti SK et al (2001) A prospective study of 2-[18 F] fluoro-2-deoxy-D-glucose/positron emission tomography scan, 99m Tc-labeled arcitumomab (CEA-scan), and blind second-look laparotomy for detecting colon cancer recurrence in patients with increasing carcinoembryonic antigen levels. Ann Surg Oncol 8:779–786
Liu F-Y et al (2005) Utility of 2-Fluoro-2-Deoxy-D-Glucose positron emission tomography in managing patients of colorectal cancer with unexplained carcinoembryonic antigen elevation at different levels. Dis Colon Rectum 48(10):1900–1912
Libutti SK et al (2001) A prospective study of 2-[18F] Fluoro-2-Deoxy-D-Glucose/Positron emission tomography scan, 99mTc-Labeled Arcitumomab (CEA-Scan), and blind second-look laparotomy for detecting colon cancer recurrence in patients with increasing carcinoembryonic antigen levels. Ann Surg Oncol 8(10):779–786
SimÓ M et al (2002) FDG-PET improves the management of patients with suspected recurrence of colorectal cancer. Nucl Med Commun 23(10):975
Shady W et al (2017) Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget 8(39):66117–66127
Kirov A et al (2019) KRAS missense mutation effects on the 18F-FDG uptake of colorectal adenocarcinoma metastases in the liver. J Nucl Med 60(supplement 1):213
Kawada K et al (2015) Relationship between 18F-FDG PET/CT scans and KRAS mutations in metastatic colorectal cancer. J Nucl Med 56(9):1322–1327
Kung BT et al (2019) An update on the role of (18)F-FDG-PET/CT in major infectious and inflammatory diseases. Am J Nucl Med Mol Imaging 9(6):255–273
Siveke JT (2018) Fibroblast-activating protein: targeting the roots of the tumor microenvironment. J Nucl Med 59(9):1412–1414
Pang Y et al (2021) Comparison of (68)Ga-FAPI and (18)F-FDG uptake in gastric, duodenal, and colorectal cancers. Radiology 298(2):393–402
Şahin E et al (2021) Comparison of (68)Ga-DOTA-FAPI and (18)FDG PET/CT imaging modalities in the detection of liver metastases in patients with gastrointestinal system cancer. Eur J Radiol 142:109867
Cuda TJ et al (2020) PET Imaging quantifying (68)Ga-PSMA-11 uptake in metastatic colorectal cancer. J Nucl Med 61(11):1576–1579
Hong YS et al (2013) 3′-Deoxy-3′-18F-fluorothymidine PET for the early prediction of response to leucovorin, 5-fluorouracil, and oxaliplatin therapy in patients with metastatic colorectal cancer. J Nucl Med 54(8):1209–1216
Mogensen MB et al (2017) FLT-PET for early response evaluation of colorectal cancer patients with liver metastases: a prospective study. EJNMMI Res 7(1):56
Anan N, Zainon R, Tamal M (2022) A review on advances in (18)F-FDG PET/CT radiomics standardisation and application in lung disease management. Insights Imaging 13(1):22
van Helden EJ et al (2018) Radiomics analysis of pre-treatment [18F]FDG PET/CT for patients with metastatic colorectal cancer undergoing palliative systemic treatment. Eur J Nucl Med Mol Imaging 45(13):2307–2317
Rahmim A et al (2019) Prognostic modeling for patients with colorectal liver metastases incorporating FDG PET radiomic features. Eur J Radiol 113:101–109
Surasi DS et al (2014) 18F-FDG PET and PET/CT patient preparation: a review of the literature. J Nucl Med Technol 42(1):5–13
Borello A et al (2021) Diagnostic performance of the FDG-PET/CT in patients with resected mucinous colorectal liver metastases. Surgeon 19(5):e140–e145
Funding
There was no funding for this review article.
Author information
Authors and Affiliations
Contributions
MZZ wrote the main manuscript text and prepared the figures.
Corresponding author
Ethics declarations
Conflict of interest
MZZ declares that he has no conflict of interest for this publication.
Informed consent
N/A to this review article. Large Language Model (ChatGPT) used solely for enhancing the language accuracy of the manuscript, not for the design or creating of ideas.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zirakchian Zadeh, M. PET/CT in assessment of colorectal liver metastases: a comprehensive review with emphasis on 18F-FDG. Clin Exp Metastasis 40, 465–491 (2023). https://doi.org/10.1007/s10585-023-10231-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-023-10231-9