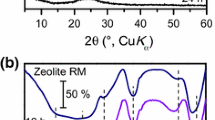
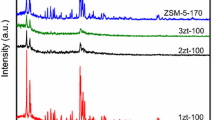
Novel silica nanoboxes were prepared by controlled dealumination of Na-X and Ca-A type zeolites using ammonium hexafluorosilicate (AHFS). The silica-richer the parent zeolite, the smaller the average pore size produced and the narrower the pore size distribution obtained. This was due to the specific reactivity of the extracting agent with the zeolite framework aluminum atoms. High temperature calcination of the dealuminated X-zeolite (ammonium form) resulted in mesoporous materials exhibiting an ink-bottle shape, a quite high surface area (330 m2/g, no micropores), an average pore diameter of 4.5 nm with a quite narrow pore size distribution (± 1.0 nm) and finally, a pore opening diameter of 3.9 nm. The latter was determined by using the nitrogen sorption isotherms (BET technique) and related pore volume data. The sorption behavior also suggested the interconnecting character of the newly created nanoboxes. The periodicity of these nanoboxes throughout the mesoporous material was clearly shown by X-ray powder diffraction at very small angles. These materials, herein called monomodal nanoboxes because of the absence of micropores in the structure, were also thermally stable. Incorporation of orthosilicate into the obtained silica nanoboxes, in accordance with the recently developed technique for pore size engineering in zeolites, led to materials with smaller pore openings but having almost the same textural properties. Solid superacidic materials were prepared by incorporating a liquid superacid (triflic acid or trifluoromethanesulfonic acid) into the silica nanoboxes using the wet impregnation technique. The maximum triflic acid loading which did not significantly affect the mesoporous framework of the materials was 24 wt%. As a reference, the maximum loading of less acidic sulfuric acid was slightly lower. All this showed the high chemical stability of the silica nanoboxes for supporting very acidic species. Temperature-programmed desorption using a combined DTA/TGA system allowed the identification of the bound phases and some liquid phase of the loaded triflic acid.
Similar content being viewed by others
References
C.T. Kresge M.E. Leonowicz W.J. Roth J.C. Vartuli J.S. Beck (1992) Nature 359 710 Occurrence Handle10.1038/359710a0 Occurrence Handle1:CAS:528:DyaK38Xms1entrs%3D
J.S. Beck J.C. Vartuli W.J. Roth M.E. Leonowicz C.T. Kresge K.D. Schmitt X.T.-W. Chu D.H. Olsen E.W. Sheppard S.B. Sheppard S.B. McCullen J.B. Higgins J.L. Schlenker (1992) J. Am. Chem. Soc. 114 10834 Occurrence Handle10.1021/ja00053a020 Occurrence Handle1:CAS:528:DyaK38Xms1entr8%3D
T.-O. Do S. Kaliaguine (2001) Angew. Chem. Int. Ed. 40 IssueID17 3248
R. Le Van Mao N.T.C. Vo B. Sjiariel L. Lee G. Denes (1992) J. Mater. Chem. 2 IssueID6 595 Occurrence Handle10.1039/jm9920200595
R. Le Van Mao N.T.C. Vo G. Denes T.S. Le (1995) J. Porous Mater. 1 175 Occurrence Handle1:CAS:528:DyaK2MXnvVaks7k%3D
R. Le Van Mao J.A. Lavigne B. Sjiariel C.H. Langford (1993) J. Mater. Chem. 3 IssueID6 679 Occurrence Handle10.1039/jm9930300679 Occurrence Handle1:CAS:528:DyaK3sXkvVCntbw%3D
R. Le Van Mao G. Denes N.T.C. Vo J.A. Lavigne S.T. Le (1995) Mat. Res. Soc. Symp. Proc. 371 123 Occurrence Handle1:CAS:528:DyaK2MXkvVKlurc%3D
C. Marcilly, in: Catatyse Acido-basique: Application au raffinage et a la petrochimie, Vol. 2 (ed.), (Technip, Paris, 2003), p. 720 and refs therein.
A.H. Janssen A.J. Koster K.P. Jong Particlede (2001) Angew. Chem. Int. Ed. 40 IssueID6 1102 Occurrence Handle10.1002/1521-3773(20010316)40:6<1102::AID-ANIE11020>3.3.CO;2-Y Occurrence Handle1:CAS:528:DC%2BD3M**sVChsrc%3D
G.W. Skeel and D.W. Breck, in: Proc. 6th Int. Zeolite Conf., Reno (U.S.A.), eds. D. Olson and A. Bisio (Butterworths, Guilford, 1984), p. 87.
D. Ohayon R. Le Van Mao D. Ciaravino H. Hazel A. Cochennec N. Rolland (2001) Appl. Catal. A: General 217 241 Occurrence Handle10.1016/S0926-860X(01)00611-1 Occurrence Handle1:CAS:528:DC%2BD3MXkvF2itb4%3D
Q.L. Wang G. Giannetto M. Guisnet (1990) Zeolites 10 301 Occurrence Handle10.1016/0144-2449(90)90084-5
P.J. Stang M.R. White (1983) Aldrichim. Acta 16 IssueID1 15 Occurrence Handle1:CAS:528:DyaL3sXktVCkt7o%3D
E.P. Barrett L.G. Joyner P.P. Halenda (1951) J. Am. Chem. Soc. 73 373 Occurrence Handle10.1021/ja01145a126 Occurrence Handle1:CAS:528:DyaG3MXjsVygsg%3D%3D
K.S.W. Sing D.H. Everett R.A.W. Haul L. Moscou R.A. Pierotti J. Rouquerol T. Siemienewska (1985) Pure Appl. Chem. 57 603 Occurrence Handle1:CAS:528:DyaL2MXhvFWrtb4%3D
R. Le Van Mao and L. Huang, in: Novel Production Methods for Ethylene, Light Hydrocarbons, and Aromatics, eds. L.F. Albright, B.L. Crynes and S. Nowak (Marcel Dekker, New York, 1992), p. 425.
R.B. Borade A. Clearfied (1995) Catal. Lett. 31 267 Occurrence Handle1:CAS:528:DyaK2MXksVegsLo%3D
J. Fripiat J. Chausson A. Jelli (1971) Chimie-Physique des Phenomenes de Surface Masson et Cie Paris 26
A.J. Lecloux, in: Catalysis Science and Technology, Vol. 2 J.R. Anderson and M. Boudart eds., (Springer-Verlag, Berlin, 1981), p. 171.
(a) J.H. de Boer, in: The Structure and Properties of Porous Materials, eds. D.H. Everett and F.S. Stone (Butterworths, London, 1958), p. 68; (b) J.H. de Boer, ibidem, p. 88.
M. Suzuki (1990) Adsorption Engineering Kodansha-Elsevier Tokyo 26
D.W. Breck (1974) Zeolite Molecular Sieves J. Wiley & Sons New York 495
Y. Liu W. Zhang T.J. Pinnavaia (2000) J. Am. Chem. Soc. 122 8791 Occurrence Handle1:CAS:528:DC%2BD3cXlvFSnsLo%3D
Y. Liu Y.J. Pinnavaia (2002) J. Mater. Chem. 12 3179 Occurrence Handle1:CAS:528:DC%2BD38XotFGht7Y%3D
T.-O. Do A. Nossov M.-A. Springuel-Huet C. Schneider J.L. Bretherton C.A. Fyfe S. Kaliaguine (2004) J. Am. Chem. Soc. 126 14324 Occurrence Handle10.1021/ja0462124 Occurrence Handle1:CAS:528:DC%2BD2cXos1Wjs7k%3D
G.A. Olah G.K. Sueya Prakash J. Sommer (1985) Superacids J. Wiley & Sons New York 36–37
J.F. Joly, in: Conversion Processes, Vol. 3 P. Leprince (ed.), (Editions Technip, Paris, 2001), pp. 287–288.
R. Le Van Mao and T.S. Le, in: Handbook of MTBE and Other Gasoline Oxygenates, eds. H. Hamid and M.A. Ali (Marcel Dekker, New York, 2004), p. 93.
K. Landskron G.A. Ozin ((26 Nov. 2004)) Science 306 1529
C & EN, (Nov. 8, 2004), p. 44.
R. Le Van Mao E. Rutinduka C. Detellier P. Gougay V. Hascoet S. Tavakoliyan S.V. Hoa T. Matsuura (1999) J. Mater. Chem. 9 IssueID3 783
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, L., Van Le Mao, R., Al-Yassir, N. et al. Silica Nanoboxes as New Nano-structured Materials: Their Secondary Synthesis from Alumina-Rich Zeolites. Catal Lett 105, 139–147 (2005). https://doi.org/10.1007/s10562-005-8683-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10562-005-8683-8