Abstract
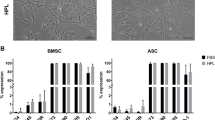
To investigate the impact of different anticoagulants and coagulants with autologous platelet-rich plasma (PRP) in order to evaluate the clinical application of PRP standardization. Bone marrow stem cells (BMSCs) were seeded into autologous PRP gel scaffolds with different anticoagulants (EDTA, heparin sodium HS, and sodium citrate SC) as well as control group (the whole blood group). Quality of PRP was evaluated and flow cytometric assay was used to detect the activity of the platelet (CD62p, PAC-1). BMSCs were also seeded into PRP with different coagulants (Thrombin, Collagen-I, ADP) as well as PRP un-activated (negative group) and L-DMEM complete culture without PRP (control group). The effects of different coagulants with PRP on proliferation, osteogenic differentiation of BMSCs were analyzed by methyl thiazolyl tetrazolium assay (MTT), ALP staining, Von Kossa staining, Confocal microscopic observation, RT-PCR and Western Blot at the morphological, cellular and molecular levels. Different anticoagulants (EDTA, HS, and SC) could affect the quality of PRP. EDTA group revealed the best quality and activity (CD62p, PAC-1). With different coagulants (Thrombin, Collagen-I and ADP) in the proliferation of BMSCs, the MTT assay showed that the proliferation of BMSCs was increased in all groups with time. On the sixth day of culture, the cell number of each PRP group was significantly higher than that in the control group (P < 0.05), while the most rapidly increasing was found in Collagen-I group. The cumulative release of growth factor (TGF-β1, PDGF) at each time point in the PRP gel of the four groups was higher than that in the control group (P < 0.05). Collagen-I was considered as the best PRP coagulant. When thrombin was used as a platelet coagulant, the release of growth factor in PRP was rapid and direct, while the release of growth factor in Collagen-I-activated PRP was sustained and slow, and the total release of ADP-activated PRP growth factors was the lowest. The study demonstrated the similar outcome in osteogenic differentiation. In terms of gene expression and western bolt, the PCR results showed that the expression levels of OCN gene and RUNX2 protein in each PRP group were higher than that in the control group (P < 0.05). Different anticoagulants caused different degrees of lysis and spontaneous activation of platelets, which lead to different quality of PRP. Compared with HS and SC, EDTA could maintain the structural integrity of platelets, reduce their spontaneous activation, and increase the release of PRP growth factors for a longer period of time, thus ensuring the biomass of PRP. In addition, different coagulants also showed different results in the proliferation as well as osteogenic differentiation of BMSCs. Compared with Thrombin and ADP, Collagen-I may be a better choice.










Similar content being viewed by others
Abbreviations
- PRP:
-
Platelet-rich plasma
- BMSCs:
-
Bone marrow stem cells
- MTT:
-
Methyl thiazolyl tetrazolium assay
- TGF:
-
Transforming growth factor
- ELISA:
-
Immunosorbent assay
- PDGF-AB:
-
Platelet-derived growth factor
- RIPA:
-
Radio immuno precipitation assay
- SDS:
-
Sodium dodecyl sulfate
- ECL:
-
Enhanced chemiluminescence
- SC:
-
Sodium citrate
- HS:
-
Heparin sodium
- CTAD:
-
Citrate-theophylline-adenine-dipyridamole
- ACD:
-
Citrate dextrose
- TRAP:
-
Thrombin receptor agonist peptide-6
References
An S, Huang X, Gao Y, Ling J, Huang Y, **ao Y (2015) FGF-2 induces the proliferation of human periodontal ligament cells and modulates their osteoblastic phenotype by affecting Runx2 expression in the presence and absence of osteogenic inducers. Int J Mol Med 36(3):705–711
Brass LF (2003) Thrombin and platelet activation. Chest 124(3):18–25
Castillo TN, Pouliot MA, Kim HJ, Dragoo JL (2011) Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med 39(2):266–271
Dohan Ehrenfest DM, Bielecki T, Jimbo R, Barbé G, Del Corso M, Inchingolo F, Sammartino G (2012) Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF). Curr Pharm Biotechnol 13(7):1145–1152
Dong BT, Tu GJ, Han YX, Chen Y (2015) Lithium enhanced cell proliferation and differentiation of mesenchymal stem cells to neural cells in rat spinal cord. Int J Clin Exp Pathol 8(3):2473–2483
Evanson JR, Guyton MK, Oliver DL, Hire JM, Topolski RL, Zumbrun SD, McPherson JC, Bojescul JA (2014) Gender and age differences in growth factor concentrations from platelet-rich plasma in adults. Mil Med 179(7):799–805
Filardo G, Kon E, Roffi A, Di Matteo B, Merli ML, Marcacci M (2015) Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc 23(9):2459–2474
Fotouhi A, Maleki A, Dolati S, Aghebati-Maleki A, Aghebati-Maleki L (2018) Platelet rich plasma, stromal vascular fraction and autologous conditioned serum in treatment of knee osteoarthritis. Biomed Pharmacother 104:652–660
Fréchette JP, Martineau I, Gagnon G (2005) Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res 84(5):434–439
Frelinger AL 3rd, Torres AS, Caiafa A, Morton CA, Berny-Lang MA, Gerrits AJ, Carmichael SL, Neculaes VB, Michelson AD (2016) Platelet-rich plasma stimulated by pulse electric fields: Platelet activation, procoagulant markers, growth factor release and cell proliferation. Platelets 27(2):128–135
Gigout T, Didelon J, Latger V, Claise C, Schooneman F, Paulus F, Stoltz JF (1998) New automatic test for the study of platelet response to variations in osmotic pressure: application in the evaluation of the viability of platelet concentrates. Transfus Clin Biol 5(5):336–345
Hou R, Yin G, An P, Wang C, Liu R, Yang Y, Yan X, Li J, Li X, Zhang K (2013) DNA methylation of dermal BMSCs in psoriasis: identification of epigenetically dysregulated genes. J Dermatol Sci 72(2):103–109
Jo CH, Lee SY, Yoon KS, Shin S (2017) Effects of platelet-rich plasma with concomitant use of a corticosteroid on tenocytes from degenerative rotator cuff tears in interleukin 1β-induced tendinopathic conditions. Am J Sports Med 45(5):1141–1150
Kececi Y, Ozsu S, Bilgir O (2014) A cost-effective method for obtaining standard platelet-rich plasma. Wounds 26(8):232–238
Koch FP, Merkel C, Al-Nawas B, Smeets R, Ziebart T, Walter C, Wagner W (2011) Zoledronate, ibandronate and clodronate enhance osteoblast differentiation in a dose dependent manner—a quantitative in vitro gene expression analysis of Dlx5, Runx2, OCN, MSX1 and MSX2. J Craniomaxillofac Surg 39(8):562–569
Koch FP, Weinbach C, Hustert E, Al-Nawas B, Wagner W (2012) GDF-5 and BMP-2 regulate bone cell differentiation by gene expression of MSX1, MSX2, Dlx5, and Runx2 and influence OCN gene expression in vitro. Int J Periodontics Restor Dent 32(3):285–293
Lam J, Marklein RA, Jimenez-Torres JA, Beebe DJ, Bauer SR, Sung KE (2017) Adaptation of a simple microfluidic platform for high-dimensional quantitative morphological analysis of human mesenchymal stromal cells on polystyrene-based substrates. SLAS Technol 22(6):646–661
Lee JS, Lee JM, Im GI (2011) Electroporation-mediated transfer of Runx2 and Osterix genes to enhance osteogenesis of adipose stem cells. Biomaterials 32(3):760–768
Lei H, Gui L, **ao R (2009) The effect of anticoagulants on the quality and biological efficacy of platelet-rich plasma. Clin Biochem 42(13-14):1452–1460
Nishiyama K, Okudera T, Watanabe T, Isobe K, Suzuki M, Masuki H, Okudera H, Uematsu K, Nakata K, Kawase T (2016) Basic characteristics of plasma rich in growth factors (PRGF): blood cell components and biological effects. Clin Exp Dent Res 2(2):96–103
Savelli S, Trombi L, D’Alessandro D, Moscato S, Pacini S, Giannotti S, Lapi S, Scatena F, Petrini M (2018) Pooled human serum: A new culture supplement for bioreactor-based cell therapies. Preliminary results. Cytotherapy 20(4):556–563
Schär MO, Diaz-Romero J, Kohl S, Zumstein MA, Nesic D (2015) Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res 473(5):1635–1643
Schlimp CJ, Solomon C, Ranucci M, Hochleitner G, Redl H, Schöchl H (2014) The effectiveness of different functional fibrinogen polymerization assays in eliminating platelet contribution to clot strength in thromboelastometry. Anesth Analg 118(2):269–276
Seabaugh KA, Thoresen M, Giguère S (2017) Extracorporeal shockwave therapy increases growth factor release from equine platelet-rich plasma in vitro. Front Vet Sci 7(4):205
Silva MA, Leite YKC, de Carvalho CES, Feitosa MLT, Alves MMM, Carvalho FAA, Neto BCV, Miglino MA, Jozala AF, de Carvalho MAM (2018) Behavior and biocompatibility of rabbit bone marrow mesenchymal stem cells with bacterial cellulose membrane. Peer J 30(6):e4656
Sugaya H, Yoshioka T, Kato T, Taniguchi Y, Kumagai H, Hyodo K, Ohneda O, Yamazaki M, Mishima H (2018) Comparative analysis of cellular and growth factor composition in bone marrow aspirate concentrate and platelet-rich plasma. Bone Marrow Res 25(2018):1549826
Tohidnezhad M, Bayer A, Rasuo B, Hock JVP, Kweider N, Fragoulis A, Sönmez TT, Jahr H, Pufe T, Lippross S (2017) Platelet-released growth factors modulate the secretion of cytokines in synoviocytes under inflammatory joint disease. Mediators Inflamm 2017:1046438
Ubilla D, Ananías J, Ortiz-Muñoz L, Irarrázaval S (2018) Is platelet-rich plasma effective for osteoarthritis. Medwave 18(3):e7215
Wang KT, Li ZL, Zhu H, Qin YY, Yang YM, Li J, Lv RJ, Liu YL, Mao N, Zhang Y (2017) Preparation of platelet-rich plasma from the white slurry and its effect on MSC proliferation. Zhongguo Shi Yan Xue Ye Xue Za Zhi 25(1):164–170
Zhang X, Wang J, Ren M, Li L, Wang Q, Hou X (2016) A novel collagen/platelet-rich plasma (COL/PRP) scaffold: preparation and growth factor release analysis. Cell Tissue Bank 17(2):327–334
Funding
This study was funded by the specialized Research Fund for Military Medicine Innovation (Grant No. 13CXZ030).
Author information
Authors and Affiliations
Contributions
ZL: Guarantor of integrity of the entire study; study concepts; study design; definition of intellectual content; manuscript review. NZ and KW: Literature research; clinical studies; experimental studies. NZ: Data acquisition; manuscript preparation; manuscript editing. NZ, KW and TL: Data analysis; statistical analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ning Zhang, Ketao Wang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, N., Wang, K., Li, Z. et al. Comparative study of different anticoagulants and coagulants in the evaluation of clinical application of platelet-rich plasma (PRP) standardization. Cell Tissue Bank 20, 61–75 (2019). https://doi.org/10.1007/s10561-019-09753-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-019-09753-y