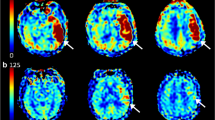
Abstract
Silent cerebral infarctions (SCI) determined by neuron specific enolase (NSE) elevation may develop more during chronic total occlusion (CTO) percutaneous coronary interventions (PCI) than non-CTO interventions. Our aim was to examine CTO and non-CTO PCIs for SCI development. 100 consecutive CTO and 100 non-CTO PCI patients were enrolled. SCI was detected by serum NSE measurements performed at baseline and 12 h after the interventions. New NSE elevations > 12 ng/mL after the procedure were counted as SCI. Post-procedural NSE levels were found to be significantly higher in the CTO PCI group and NSE positivity was more prevalent in the CTO PCI group (56 (56%) vs. 31 (31%), p < 0.001), but PCI of CTOs did not independently increase risk of SCI (OR: 2.39 (0.85–6.73), p: 0.10). Patients who developed SCI after PCI had the characteristics of tough PCI interventions. In the multivariate analysis, two parameters were found to be independently associated with SCI development, namely more contrast volume (OR: 1.014 (1.005–1.023), p: 0.003) and longer procedural time (OR: 1.030 (1.010–1.051), p: 0.003). It has been firstly demonstrated in the literature that CTO PCIs, by its nature, have increased rates of SCI when compared to non-CTO PCIs but presence of a CTO was not an independent predictor of SCI. Mainly, procedural characteristics of the PCIs, especially longer procedural times and more contrast consumption, observed more in CTO PCIs, have been found to be independently associated with elevations of plasma NSE levels.

Similar content being viewed by others
References
Jeroudi OM, Alomar ME, Michael TT et al (2014) Prevalence and management of coronary chronic total occlusions in a tertiary Veterans Affairs hospital. Catheter Cardiovasc Interv 84:637–643
Werner GS, Martin-Yuste V, Hildick-Smith D et al (2018) A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J 39:2484–2493
Mashayekhi K, Nuhrenberg TG, Toma A et al (2018) A randomized trial to assess regional left ventricular function after stent implantation in chronic total occlusion: the REVASC trial. JACC Cardiovasc Interv 11:1982–1991
Neumann FJ, Sousa-Uva M, Ahlsson A et al (2019) 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 40:87–165
Brilakis ES, Banerjee S, Karmpaliotis D et al (2015) Procedural outcomes of chronic total occlusion percutaneous coronary intervention: a report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv 8:245–253
Maeremans J, Walsh S, Knaapen P et al (2016) The hybrid algorithm for treating chronic total occlusions in Europe: the RECHARGE registry. J Am Coll Cardiol 68:1958–1970
Galassi AR, Sianos G, Werner GS et al (2015) Retrograde recanalization of chronic total occlusions in Europe: procedural, in-hospital, and long-term outcomes from the multicenter ERCTO registry. J Am Coll Cardiol 65:2388–2400
Barone FC, Clark RK, Price WJ et al (1993) Neuron-specific enolase increases in cerebral and systemic circulation following focal ischemia. Brain Res 623:77–82
Stevens H, Jakobs C, de Jager AE, Cunningham RT, Korf J (1999) Neuron-specific enolase and N-acetyl-aspartate as potential peripheral markers of ischaemic stroke. Eur J Clin Invest 29:6–11
Anand N, Stead LG (2005) Neuron-specific enolase as a marker for acute ischemic stroke: a systematic review. Cerebrovasc Dis 20:213–219
Goksuluk H, Gulec S, Ozcan OU et al (2016) Usefulness of neuron-specific enolase to detect silent neuronal ischemia after percutaneous coronary intervention. Am J Cardiol 117:1917–1920
Avdibegovic E, Becirovic E, Salimbasic Z, Hasanovic M, Sinanovic O (2007) Cerebral cortical atrophy and silent brain infarcts in psychiatric patients. Psychiatr Danub 19:49–55
Wright CB, Festa JR, Paik MC et al (2008) White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke 39:800–805
Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM (2003) Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 348:1215–1222
Bokura H, Kobayashi S, Yamaguchi S et al (2006) Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: a prospective cohort study. J Stroke Cerebrovasc Dis 15:57–63
Liebetrau M, Steen B, Hamann GF, Skoog I (2004) Silent and symptomatic infarcts on cranial computerized tomography in relation to dementia and mortality: a population-based study in 85-year-old subjects. Stroke 35:1816–1820
Thygesen K, Alpert JS, Jaffe AS et al (2018) Fourth universal definition of myocardial infarction (2018). Circulation 138:e618–e651
Morino Y, Abe M, Morimoto T et al (2011) Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicentre CTO registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv 4:213–221
Karalis DG, Quinn V, Victor MF et al (1996) Risk of catheter-related emboli in patients with atherosclerotic debris in the thoracic aorta. Am Heart J 131:1149–1155
Spina R, Simon N, Markus R, Muller DW, Kathir K (2017) Contrast-induced encephalopathy following cardiac catheterization. Catheter Cardiovasc Interv 90:257–268
Göksülük H, Güleç S, Özyüncü N et al (2018) Comparison of frequency of silent cerebral ınfarction after coronary angiography and stenting with transradial versus transfemoral approaches. Am J Cardiol 122:548–553
Hassell ME, Nijveldt R, Roos YB et al (2013) Silent cerebral infarcts associated with cardiac disease and procedures. Nat Rev Cardiol 10:696–706
Ghanem A, Müller A, Nahle CP et al (2010) Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol 55:1427–1432
Bendszus M, Reents W, Franke D et al (2002) Brain damage after coronary artery bypass grafting. Arch Neurol 59:1090–1095
Gaita F, Caponi D, Pianelli M et al (2010) Radiofrequency catheter ablation of atrial fibrillation: a cause of silent thromboembolism? Magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation 122:1667–1673
Deveci OS, Celik AI, Ikikardes F et al (2016) The incidence and the risk factors of silent embolic cerebral ınfarction after coronary angiography and percutaneous coronary ınterventions. Angiology 67:433–437
Aykan AÇ, Gökdeniz T, Bektaş H et al (2016) Assessment of silent neuronal ınjury following coronary angiography and ıntervention in patients with acute coronary syndrome. Clin Appl Thromb Hemost 22:52–59
Oh SH, Lee JG, Na SJ, Park JH, Choi YC, Kim WJ (2003) Prediction of early clinical severity and extent of neuronal damage in anterior-circulation ınfarction using the ınitial serum neuron-specific enolase level. Arch Neurol 60:37–41
Hill MD, Jackowski G, Bayer N, Lawrence M, Jaeschke R (2000) Biochemical markers in acute ischemic stroke. CMAJ 162:1139–1140
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arslan, U., Yenerçağ, M., Erdoğan, G. et al. Silent cerebral infarction after percutaneous coronary intervention of chronic total occlusions (CTO) and non-CTOs. Int J Cardiovasc Imaging 36, 2107–2113 (2020). https://doi.org/10.1007/s10554-020-01939-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-01939-w