Abstract
Purpose
There have been very few reports of secondary malignancies after breast cancer treatment in Asia, particularly in Japan. This study aimed to evaluate the risk of secondary malignancies after radiotherapy (RT) in Japanese breast cancer patients.
Methods
This single-center retrospective study included patients who underwent RT between July 1961 and September 2006 for postoperative breast cancer. A total of 702 patients with a follow-up period of more than 5 years were analyzed. All malignancies observed at more than 5 years after the start of RT were defined as secondary malignancies. To calculate the relative risk (RR) of secondary malignancies, we applied data from the National Cancer Center in Japan.
Results
The median observation period was 9.7 (interquartile range 7.1–18.2) years. The cumulative person-years of observation were 6879.4. The RR of contralateral breast cancer increased by 1.85-fold (95% confidence interval [CI] 1.05–3.26) among patients compared with that among the general population; however, the difference was not significant (p = 0.053). The RR of secondary malignancies other than breast cancer increased by 2.71-fold (95% CI 1.99–3.70, p < 0.001) among the patients compared with the general population. Even when only malignancies detected more than 10 years after RT were defined as secondary malignancies, the RR of secondary malignancies other than breast cancer was 1.91 (95% CI 1.33–2.73, p < 0.001).
Conclusion
The incidence of secondary malignancies after RT may be somewhat higher in Japanese patients with breast cancer than in the general population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a common cancer in many countries. In Japan, approximately 94,000 patients were diagnosed with breast cancer, and more than 15,000 individuals died of breast cancer in 2021 [1]. Radiotherapy (RT) plays a vital role in the treatment strategy of breast cancer together with surgery and systemic treatments: specifically, its role is to prevent local and regional lymph node recurrences. The effect of RT on local control is observed at a constant rate, regardless of age, tumor factors, or concomitant systemic treatment, being more effective in patients with a higher risk of local recurrence [2]. Moreover, RT improves the survival rate after breast-conserving surgery regardless of the presence of axillary lymph node metastasis [2]. Therefore, it is highly recommended unless the patient is pregnant or has a specific genetic disorder [3, 4].
Breast cancer has a long-term prognosis if treated appropriately. Hence, proper management and prevention of adverse events are essential. Dermatitis, subcutaneous tissue inflammation, and pneumonia are well-known adverse events in the acute phase after RT [5, 6]. Meanwhile, late adverse events include upper extremity edema, cardiac disease, and secondary malignancies [7,8,9]. In particular, secondary malignancies after breast cancer treatment can be fatal. Therefore, secondary malignancies, including contralateral breast cancer and other malignancies, after breast cancer treatment have been studied. Taylor et al. reported that RT increased the relative risk (RR) of breast cancer in the contralateral breast by 1.20-fold (95% confidence interval [CI] 1.08–1.33) [8]. A meta-analysis conducted by the Early Breast Cancer Trialists’ Collaborative Group also reported that RT increased the RR of breast cancer in the contralateral breast by 1.18-fold [2]. RT for postoperative breast cancer is also associated with an increased risk of secondary malignancies other than breast cancer. Taylor et al. reported that RT increased the RR of secondary malignancies other than breast cancer to 1.23 (95% CI 1.12–1.36), and Grantzau and Overgaard also reported an increase in the RR to 1.22 (95% CI 1.06–1.41) [8, 9]. Thus, postoperative RT for breast cancer increases the risk of secondary malignancies, although to a small degree.
Calip et al. reported that the risk of secondary malignancies differs according to race and ethnicity [10]. They assessed the risk of secondary malignancy among breast cancer survivors for each case group defined by race/ethnicity (non-Hispanic White, Black, Hispanic, and Asian/Pacific Islander) and found that Black and Asian/Pacific Islander women had a higher risk of secondary malignancies. Terao et al. recently reported that the genomic locations of mutations in their respective hematopoietic clones differed significantly between Japanese and European individuals and that these differences predicted the relative rates of chronic lymphocytic leukemia (which is more common in Europeans) and T-cell leukemia (which is more common in Japanese individuals) in these populations [11]. Although their study focused on leukemia and not solid tumors, the findings suggest the need for more research on the differences in the risk of secondary malignancies among racial groups. However, there are still very few reports on secondary malignancies after breast cancer treatment in Asia, particularly in Japan. Hence, this study aimed to evaluate the risk of secondary malignancies after RT in Japanese patients with breast cancer.
Materials and methods
Study design
This single-center retrospective study included patients who underwent RT between July 1961 and September 2006 for postoperative breast cancer at the National Institutes for Quantum Science and Technology (QST) Hospital. All RT procedures were performed in accordance with the standards of care for each decade [12]. The RT equipment for each age group was as follows: Vickers-Armstrong 6 MeV (1963–1973), Mitsubishi ML-15M2 (1973–1985), Mitsubishi ML-10X (1985–1996), Varian Clinac2100C (1997–2005), and Varian Clinac21EX (2005–2006). All RTs involved X-rays alone or a combination of X-rays and electron beams. No patients received intensity-modulated RT (IMRT). Linear accelerators delivered all RTs with 6 MV or 4 MV X-rays and ≥ 6 MeV electrons. In terms of irradiation fields, there were mixed irradiation cases of the conserved breast, chest wall, supraclavicular fossa, or parasternal/intermammary lymph node area. One thousand six hundred twenty-one patients were included in the study, out of whom 702 that had a follow-up period of more than 5 years were analyzed. All malignancies observed at more than 5 years after the start of RT were defined as secondary malignancies. This retrospective study was approved by the Institutional Ethics Review Board (N21-015). The need for written informed consent was waived owing to the retrospective nature of the study. Instead, a document for an opt-out policy was uploaded to the webpage of the QST Hospital, which allowed any of the patients and their families to refuse to be included in the study.
Data collection
We collected data on the patients’ age at the time of RT initiation, dose of RT, date of RT initiation, date of the second cancer diagnosis, date of death or the last visit from the QST database, and medical records. As previously reported, we confirmed the information on secondary malignancy by checking medical records, radiology reports, surgical records, and pathology reports [13]. Considering the appropriate incubation period, as reported by Grantzau et al., secondary malignancies were defined as all malignancies observed for the first time at 5 years after the initiation of RT [9]. Only those lesions with a histological type differing from that of the initial breast cancer, and with no evidence of recurrence, were classified as secondary malignancies.
The patients were followed-up every 6 months until 5 years after RT and once a year after that. Patients who did not undergo face-to-face follow-up were sent a yearly questionnaire with specific questions on breast cancer recurrence, adverse events after treatment, and secondary malignancy development. Additional information on secondary malignancies was gathered by consulting other doctors, hospitals, and patients or their families. With the approval of the Ministry of Justice, missing patient data were supplemented from the Japanese nationwide registry, including the date and cause of death. The observation period started at the date of initiation of RT for breast cancer and ended at the date of death or last visit.
Data analysis
To calculate the RR of secondary malignancies in the cohort, we applied data from the National Cancer Center in Japan, which shows site-specific cancer incidence rates in the general female population in Japan [14]. The RR of secondary malignancies was calculated by comparing the incidence rate of cancer in the general population with that of secondary malignancies per person-year in the study cohort. For the cancer incidence rates in the general population, age-adjusted cancer incidence rates were used to eliminate the effect of increased cancer incidence rates with aging. The Mann–Whitney U test or Welch’s t test was employed for continuous variables and the chi-square test with Yates’ adjustment for nominal variables. The cumulative incidence rate of secondary malignancies was calculated using the Kaplan–Meier method. The log-rank test was used to compare the cumulative incidence rates. All statistical tests were two-sided, and all comparisons were considered statistically significant when the p-value was less than 0.05. The Statistical Package for the Social Sciences for Macintosh, version 27.0, (IBM Inc., Armonk, NY, USA) was used for all statistical analyses.
Results
Among the 702 patients analyzed in the study, the median age at the start of RT was 51 years (interquartile range [IQR] 44–61). The median observation period was 9.7 (IQR 7.1–18.2) years (Table 1). The cumulative person-years of observation were 6879.4. Of the 702 patients, 60 secondary malignancies were observed in 57 patients. At the onset of secondary malignancies, the median years was 14.4 years (IQR 9.0–24.2). There was no notable bias in calendar years when RT was conducted. The sites and number of secondary malignancies are listed in Table 2.
Table 3 lists the RRs of secondary malignancies by sites after RT. The RR of all secondary malignancies was 2.463 (95% CI 1.877–3.231), which increased significantly over the general female population (p < 0.001). RT increased the RR of breast cancer in the contralateral breast by 1.85-fold (95% CI 1.05–3.26) among the patients compared with the general population, although no significant difference was observed (p = 0.053). The RR of secondary malignancies other than breast cancer increased by 2.71-fold (95% CI 1.99–3.70, p < 0.001) among the patients compared with the general population. Even when only malignancies detected at more than 10 years after RT were defined as secondary malignancies, the RR of secondary malignancies other than breast cancer was 1.91 (95% CI 1.33–2.73, p < 0.001).
In the analysis by site of secondary malignancy, the RRs of secondary lung, colonic, rectal, stomach, ovarian, esophageal, and bladder carcinomas increased significantly. The secondary malignancies of the lung, esophagus, and bladder all developed at 10 years after RT. All patients with secondary ovarian carcinoma were less than 50 years of age at the start of RT. No significant differences in the RRs were observed for secondary thyroid, pancreatic, kidney, tongue, and skin malignancies among the patients compared with those in the general population.
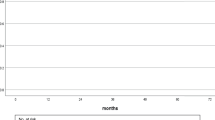
The 10-year and 20-year cumulative incidence rates of secondary malignancies were 3.6% (95% CI 1.9–5.3) and 12.4% (95% CI 8.4–16.3), respectively. When age was dichotomized by median age (51 years), there was no significant association between age and the occurrence of secondary malignancies (p = 0.130). When the RT period was dichotomized (1961–1984 vs. 1984–2006), a higher incidence rate of secondary malignancies was observed in the group treated more recently (p < 0.001). The 10-year and 20-year cumulative incidence rates of contralateral breast cancer were 0.4% (95% CI 0.0–0.7) and 4.3% (95% CI 1.7–6.9), respectively. The 10-year and 20-year cumulative incidence rates of malignancies other than contralateral breast cancer were 3.1% (95% CI 1.5–4.7) and 8.4% (95% CI 5.1–11.7), respectively. Although contralateral breast cancer appeared by approximately 20 years after RT, the incidence rate of secondary malignancies other than contralateral breast cancer gradually increased over time. The incidence rate of contralateral breast cancer and other secondary malignancies were significantly different (p < 0.001) (Fig. 1).
Discussion
To the best of our knowledge, this is the first long-term observational study on secondary malignancies after RT for breast cancer in Japan. Although this was a single-center study, it analyzed more than 700 cases and included an observational period of approximately 6800 person-years, which can be considered a medium-scale study of secondary malignancy. Although the number of person-years was inferior to that in studies using large databases, the advantage of this study was that it provided highly accurate information on secondary malignancy by utilizing the characteristics of a single-center study. As in previous studies, our study validated the finding that RT after breast cancer surgery increases the risk of secondary malignancies in the Japanese population [2, 8, 9].
Several non-randomized studies have indicated that RT increases the risk of contralateral secondary breast cancer [15, 16]. In addition, Taylor et al. confirmed that postoperative breast cancer RT significantly increases the risk of contralateral breast cancer [8]. In support of these results, our study also implied that RT increased the RR of secondary breast cancer in the contralateral breast by 1.85-fold (95% CI 1.05–3.26); however, the increase was not significant (p = 0.053). This discrepancy is not simply attributed to the relatively small number of person-years but may be attributed to the longer follow-up period in our study. Taylor et al. also showed that the risk of contralateral breast cancer peaked at 5–9 years post-treatment and then declined as the observation period increased [8]. Accordingly, the incidence rate of contralateral breast cancer increased progressively up to 15 years after RT but was almost nonexistent thereafter (Fig. 1). Thus, the risk of contralateral breast cancer after RT for breast cancer should also consider the time lapse after the primary treatment.
Our study showed that RT for breast cancer increased the RR of secondary malignancies other than breast cancer by 2.71-fold among the patients compared with the general female population in Japan (95% CI 1.99–3.70, p < 0.001). Even when only malignancies detected at more than 10 years after RT were defined as secondary malignancies, the RR of secondary malignancies other than breast cancer was 1.91 (95% CI 1.33–2.73, p < 0.001). Previous meta-analyses demonstrated that the RR of secondary malignancies other than breast cancer was 1.22–1.23 [8, 9]. It should be noted that these studies seem to compare breast cancer patients with and without RT, whereas the present study compares breast cancer patients with the general population; thus, a simple comparison is not appropriate because of the different study methods used. The higher RR found may be related to other factors. There may be racial differences in secondary malignancies after breast cancer treatment, as reported by Calip et al. [10]. Besides, the genetic or biological attributes of each racial/ethnic group may explain the distribution of secondary cancer risk [17]. In line with this, Totoki et al. reported differences in genes encoding metabolic enzymes, including chromatin remodelers, in Asian and European ancestry populations [18]. In discussions of the risk of secondary malignancies, research focusing on such somatic mutations will become even more critical in future. Furthermore, socioeconomic, behavioral, and lifestyle factors may affect the risk of develo** secondary malignancies. Further studies comprehensively including these factors are warranted to determine whether there is a difference in the risk of secondary malignancies between races.
Zablotska et al. reported an increased risk of lung and esophageal secondary cancers after breast cancer treatment; in both studies, only RT after mastectomy was significantly associated with increased risk [19, 20]. Our results are consistent with these findings. Morton et al. found that in female patients surviving breast cancer for more than 5 years, the excess odds ratio for secondary esophageal cancer increased linearly by 9% for each additional one Gy of tumor site dose [21]. Grantzau et al. reported that the rate of secondary lung cancer increased linearly, with 8.5% per delivered Gray to the lung, among breast cancer survivors for more than 5 years [22]. Therefore, it is likely that there is a dose-dependent increase in the risk of secondary cancer. Meanwhile, an increased incidence of secondary thyroid cancer was reported in patients with childhood cancer who received RT [23]. However, there was no excess risk of secondary cancers of the oral cavity and thyroid in the general population, which are relatively close to the irradiation fields in our study. Regarding secondary thyroid cancer, the risk may be higher in patients who received RT during childhood; however, there is probably no evidence that the risk of thyroid cancer increases as secondary cancer after RT in adults.
Herein, secondary malignancies at sites far from the irradiation field were also identified. The five patients with secondary ovarian cancer were less than 50 years old at the start of RT and may have had hereditary breast and ovarian cancer syndromes [24]. Lifestyle factors, such as alcohol consumption and smoking habits, and the effects of breast cancer chemotherapy on secondary malignancies in some patients should also be considered [25,26,27]. Thus, it is difficult to accurately investigate RT-induced secondary malignancies in a true sense. Best et al. reported that two variants on chromosome 6q21 were linked to secondary cancers in survivors of Hodgkin’s lymphoma treated with RT but not in adults [28]. Since there may be complex confounding of various factors in the development of secondary cancers, it would be desirable to use genetic mutations to assess the risk of cancer from radiation in future.
When the RT period was dichotomized (1961–1984 vs. 1984–2006), a higher incidence of secondary malignancies was observed in the group treated more recently (p < 0.001). Compared with conventional RT, IMRT increases the incidence rate of secondary cancers by 1.7-fold because it uses more treatment fields and exposes more normal tissue to low-dose radiation [29]. However, IMRT was not used in this study, and the reason for the higher incidence rate of secondary cancers in more recently treated patients was unclear. Technological advances in diagnostic medical devices may have led to the early detection of secondary cancers. Unfortunately, our study did not cover the details of the medical devices used to screen for secondary malignancies; therefore, further research that would consider this is necessary. As shown in Fig. 1, contralateral breast cancer appeared by approximately 20 years after RT, while the incidence rate of secondary malignancies other than contralateral breast cancer gradually increased over time. Accordingly, it is necessary to provide medical care and educate patients regarding the long-term development of secondary malignancies after RT for breast cancer.
Our study has several limitations. First, it was a single-center, retrospective study. This is a common problem in other studies on secondary malignancies, and prospective studies are needed to accurately identify the incidence rate of secondary malignancies. Second, we did not have any information on genetic factors, family history, adjuvant chemotherapy, or lifestyle factors that could influence the risk of secondary malignancies. Comprehensive studies that consider these factors are warranted to truly identify radiation-induced cancers. In addition, it would be necessary to mention the observation period. The median observation period in this study was 9.7 years, which is a moderate duration for a study of secondary malignancies. There was no significant difference in the occurrence of secondary malignancies when age was dichotomized by median age in this study. However, re-evaluation after long-term observation would be warranted because secondary malignancies still occur 10 years after treatment.
In conclusion, we found that the incidence of secondary malignancies after RT may be somewhat higher in Japanese patients with breast cancer than in the general population. Further large-scale studies are warranted to validate our findings.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to institutional policy but are available from the corresponding author upon reasonable request.
References
Cancer Statistics. Cancer Information Service, National Cancer Center, Japan. https://ganjoho.jp/reg_stat/statistics/stat/short_pred_en.html. Accessed 12 Feb 2022
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V et al (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366(9503):2087–2106
Loibl S, von Minckwitz G, Gwyn K, Ellis P, Blohmer JU, Schlegelberger B, et al (2006) Breast carcinoma during pregnancy. International recommendations from an expert meeting. Cancer 106(2):237–246
Tung NM, Boughey JC, Pierce LJ, Robson ME, Bedrosian I, Dietz JR et al (2020) Management of hereditary breast cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J Clin Oncol 38(18):2080–2106
Ogo E, Komaki R, Fujimoto K, Uchida M, Abe T, Nakamura K et al (2008) A survey of radiation-induced bronchiolitis obliterans organizing pneumonia syndrome after breast-conserving therapy in Japan. Int J Radiat Oncol Biol Phys 71(1):123–131
Lingos TI, Recht A, Vicini F, Abner A, Silver B, Harris JR (1991) Radiation pneumonitis in breast cancer patients treated with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys 21(2):355–360
Shaitelman SF, Chiang YJ, Griffin KD, DeSnyder SM, Smith BD, Schaverien MV et al (2017) Radiation therapy targets and the risk of breast cancer-related lymphedema: a systematic review and network meta-analysis. Breast Cancer Res Treat 162(2):201–215
Taylor C, Correa C, Duane FK, Aznar MC, Anderson SJ, Bergh J, et al (2017) Early Breast Cancer Trialists’ Collaborative Group. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol 35(15):1641–1649
Grantzau T, Overgaard J (2015) Risk of second non-breast cancer after radiotherapy for breast cancer: a systematic review and meta-analysis of 762,468 patients. Radiother Oncol 114(1):56–65
Calip GS, Law EH, Ko NY (2015) Racial and ethnic differences in risk of second primary cancers among breast cancer survivors. Breast Cancer Res Treat 151(3):687–696
Terao C, Suzuki A, Momozawa Y, Akiyama M, Ishigaki K, Yamamoto K et al (2020) Chromosomal alterations among age-related haematopoietic clones in Japan. Nature 584(7819):130–135
Clinical Practice Guideline in Oncology, Breast Cancer. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419. Accessed 12 Feb 2022
Mohamad O, Tabuchi T, Nitta Y, Nomoto A, Sato A, Kasuya G et al (2019) Risk of subsequent primary cancers after carbon ion radiotherapy, photon radiotherapy, or surgery for localised prostate cancer: a propensity score-weighted, retrospective, cohort study. Lancet Oncol 20(5):674–685
Cancer Statistics. Cancer Information Service, National Cancer Center, Japan (National Cancer Registry, Ministry of Health, Labour and Welfare). 2. Incidence, 1) National Cancer Registry. https://ganjoho.jp/reg_stat/statistics/data/dl/en.html. Accessed 12 Feb 2022
Hooning MJ, Aleman BM, Hauptmann M, Baaijens MH, Klijn JG, Noyon R et al (2008) Roles of radiotherapy and chemotherapy in the development of contralateral breast cancer. J Clin Oncol 26:5561–5568
Berrington de Gonzalez A, Curtis RE, Gilbert E, Berg CD, Smith SA, Stovall M et al (2021) Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer 102:220–226
Ademuyiwa FO, Olopade OI (2003) Racial differences in genetic factors associated with breast cancer. Cancer Metastasis Rev 22(1):47–53
Totoki Y, Tatsuno K, Covington KR, Ueda H, Creighton CJ, Kato M et al (2014) Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet 46(12):1267–1273
Zablotska LB, Neugut AI (2003) Lung carcinoma after radiation therapy in women treated with lumpectomy or mastectomy for primary breast carcinoma. Cancer 97:1404–1411
Zablotska LB, Chak A, Das A, Neugut AI (2005) Increased risk of squamous cell esophageal cancer after adjuvant radiation therapy for primary breast cancer. Am J Epidemiol 161:330–337
Morton LM, Gilbert ES, Hall P, Andersson M, Joensuu H, Vaalavirta L et al (2012) Risk of treatment-related esophageal cancer among breast cancer survivors. Ann Oncol 23:3081–3091
Grantzau T, Thomsen MS, Væth M, Overgaard J (2014) Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother Oncol 111:366–373
de Vathaire F, Hardiman C, Shamsaldin A, Campbell S, Grimaud E, Hawkins M et al (1999) Thyroid carcinomas after irradiation for a first cancer during childhood. Arch Intern Med 159(22):2713–2719
Quinn GP, Pal T, Murphy D, Vadaparampil ST, Kumar A (2012) High-risk consumers’ perceptions of preimplantation genetic diagnosis for hereditary cancers: a systematic review and meta-analysis. Genet Med 14(2):191–200
Pesch B, Kendzia B, Gustavsson P, Jöckel KH, Johnen G, Pohlabeln H et al (2012) Cigarette smoking and lung cancer–relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer 131:1210–1219
Polednak AP (2008) Estimating the number of U.S. incident cancers attributable to obesity and the impact on temporal trends in incidence rates for obesity-related cancers. Cancer Detect Prev 32:190–199
Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES et al (2013) Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975–2008. Blood 121(15):2996–3004
Best T, Li D, Skol AD, Kirchhoff T, Jackson SA, Yasui Y et al (2011) Variants at 6q21 implicate PRDM1 in the etiology of therapy-induced second malignancies after Hodgkin’s lymphoma. Nat Med 17(8):941–943
Hall EJ, Wuu CS (2003) Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys 56:83–88
Acknowledgements
We thank Mr. Ichiro Tsuruoka and Ms. Tomoko Takahashi for their assistance during our study.
Funding
This work was supported by management expense grants from the National Institutes for Quantum Science and Technology, Chiba, Japan. No grants were obtained from external sources.
Author information
Authors and Affiliations
Contributions
NO contributed to conceptualization, data curation, methodology, formal analysis, investigation, writing—original draft, and visualization. KK contributed to conceptualization, data curation, methodology, project administration, supervision, writing, review, editing, and supervision. YN contributed to resources and investigation. YM, KM, and MW contributed to resources. HT contributed to supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the National Institutes for Quantum Science and Technology (N21-015. 13 Nov 2021).
Informed consent
The need for written informed consent was waived because of the retrospective nature of the study. Instead, a document for an opt-out policy was uploaded to the webpage of the QST Hospital, which allowed any of the patients and their families to refuse to be included in the study.
Consent to publish
The need for written informed consent was waived because of the retrospective nature of the study. The document for an opt-out policy was uploaded on the webpage of the QST Hospital, which included the study outline and the policy that all data could be used as tables and figures. Our study allowed all patients and their families to refuse to be included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okonogi, N., Karasawa, K., Nitta, Y. et al. Risk of secondary malignancy after radiotherapy for breast cancer: long-term follow-up of Japanese patients with breast cancer. Breast Cancer Res Treat 194, 561–567 (2022). https://doi.org/10.1007/s10549-022-06644-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06644-x