Abstract
Purpose
Despite the recent expansion in the use of immunotherapy for many cancer types, it is still not a standard treatment for breast cancer. Identifying differences in the immune systems of breast cancer patients compared to healthy women might provide insight into potential targets for immunotherapy and thus may assist its clinical implementation.
Methods
Multi-colour flow cytometry was used to investigate myeloid and lymphoid populations in the peripheral blood of breast cancer patients (n = 40) and in the blood of healthy age-matched women (n = 25). We additionally performed functional testing to identify immune suppressive mechanisms used by circulating CD14+ myeloid cells from breast cancer patients.
Results
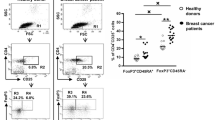
Our results show that breast cancer patients have significantly elevated frequencies of cells with the monocytic myeloid-derived suppressor cell (mMDSC) phenotype CD14+ HLA-DR−/low compared with healthy women (p < 0.01). We also observed higher levels of earlier differentiated T cells and correspondingly lower levels of T cells in later stages of differentiation (p < 0.05). These disease-associated differences could already be detected in early-stage breast cancer patients in stages 1 and 2 (n = 33 of 40) (p < 0.05). Levels of circulating T cells correlated with certain clinical features and with patient age (p < 0.05). Functional tests showed that CD14+ myeloid cells from breast cancer patients more potently suppressed autologous T cell proliferation than CD14+ cells from healthy women (p < 0.01). Subsequent investigation determined that suppression was mediated in part by reactive oxygen species, because inhibiting this pathway partially restored T cell proliferation (p < 0.01).
Conclusion
Our results highlight the potential importance of cells with mMDSC phenotypes in breast cancer, identifiable already at early stages of disease. This may provide a basis for identifying possible new therapeutic targets to enhance anti-cancer immunity.



Similar content being viewed by others
References
Breast cancer estimated incidence, mortality and prevalence worldwide in 2012. 2012. http://globocan.iarc.fr/old/FactSheets/cancers/breast-new.asp
Aerts JG, Hegmans JP (2013) Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res 73(8):2381–2388. https://doi.org/10.1158/0008-5472.CAN-12-3932
Malas S, Harrasser M, Lacy KE, Karagiannis SN (2014) Antibody therapies for melanoma: new and emerging opportunities to activate immunity (Review). Oncol Rep 32(3):875–886. https://doi.org/10.3892/or.2014.3275
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, Investigators K- (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372(21):2018–2028. https://doi.org/10.1056/NEJMoa1501824
Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, Margolin KA, Plimack ER, Lambert AM, Waxman IM, Hammers HJ (2015) Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 33(13):1430–1437. https://doi.org/10.1200/JCO.2014.59.0703
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: big 02-98. J Clin Oncol 31(7):860–867. https://doi.org/10.1200/JCO.2011.41.0902
Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, Wolff AC, Wood WC, Davidson NE, Sledge GW, Sparano JA, Badve SS (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: eCOG 2197 and ECOG 1199. J Clin Oncol 32(27):2959–2966. https://doi.org/10.1200/JCO.2013.55.0491
Mittendorf EA, Clifton GT, Holmes JP, Schneble E, van Echo D, Ponniah S, and Peoples GE (2014) Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol 25(9):1735–1742. https://doi.org/10.1093/annonc/mdu211
Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L (2016) Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol 34(21):2460–2467. https://doi.org/10.1200/JCO.2015.64.8931
Emens L, Braiteh F, Cassier P, DeLord J, Eder J, Shen X, **ao Y, Wang Y, Hegde P, Chen D, Krop I (2015) Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer. Cancer Res. https://doi.org/10.1158/1538-7445
Domschke C, Schneeweiss A, Stefanovic S, Wallwiener M, Heil J, Rom J, Sohn C, Beckhove P, Schuetz F (2016) Cellular immune responses and immune escape mechanisms in breast cancer: determinants of immunotherapy. Breast Care (Basel). 11(2):102–107. https://doi.org/10.1159/000446061
Baniyash M (2016) Myeloid-derived suppressor cells as intruders and targets: clinical implications in cancer therapy. Cancer Immunol Immunother 65(7):857–867. https://doi.org/10.1007/s00262-016-1849-y
Heine A, Schilling J, Grunwald B, Kruger A, Gevensleben H, Held SA, Garbi N, Kurts C, Brossart P, Knolle P, Diehl L, Hochst B (2016) The induction of human myeloid derived suppressor cells through hepatic stellate cells is dose-dependently inhibited by the tyrosine kinase inhibitors nilotinib, dasatinib and sorafenib, but not sunitinib. Cancer Immunol Immunother 65(3):273–282. https://doi.org/10.1007/s00262-015-1790-5
Rodriguez PC, Ochoa AC (2008) Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev 222:180–191. https://doi.org/10.1111/j.1600-065X.2008.00608.x
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10):R453–R462. https://doi.org/10.1016/j.cub.2014.03.034
Yoshimura A, Muto G (2011) TGF-beta function in immune suppression. Curr Top Microbiol Immunol 350:127–147. https://doi.org/10.1007/82_2010_87
Kortylewski M, Yu H (2008) Role of Stat3 in suppressing anti-tumor immunity. Curr Opin Immunol 20(2):228–233. https://doi.org/10.1016/j.coi.2008.03.010
Shipp C, Speigl L, Janssen N, Martens A, Pawelec G (2016) A clinical and biological perspective of human myeloid-derived suppressor cells in cancer. Cell Mol Life Sci 73(21):4043–4061. https://doi.org/10.1007/s00018-016-2278-y
Kalathil SG, Thanavala Y (2016) High immunosuppressive burden in cancer patients: a major hurdle for cancer immunotherapy. Cancer Immunol Immunother 65(7):813–819. https://doi.org/10.1007/s00262-016-1810-0
Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ (2009) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 58(1):49–59. https://doi.org/10.1007/s00262-008-0523-4
Choi J, Suh B, Ahn YO, Kim TM, Lee JO, Lee SH, Heo DS (2012) CD15 +/CD16low human granulocytes from terminal cancer patients: granulocytic myeloid-derived suppressor cells that have suppressive function. Tumour Biol 33(1):121–129. https://doi.org/10.1007/s13277-011-0254-6
Yu J, Wang Y, Yan F, Zhang P, Li H, Zhao H, Yan C, Yan F, Ren X (2014) Noncanonical NF-kappaB activation mediates STAT3-stimulated IDO upregulation in myeloid-derived suppressor cells in breast cancer. J Immunol. 193(5):2574–2586. https://doi.org/10.4049/jimmunol.1400833
Toor SM, Syed Khaja AS, El Salhat H, Faour I, Kanbar J, Quadri AA, Albashir M, Elkord E (2017) Myeloid cells in circulation and tumor microenvironment of breast cancer patients. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-017-1977-z
Bergenfelz C, Larsson AM, von Stedingk K, Gruvberger-Saal S, Aaltonen K, Jansson S, Jernstrom H, Janols H, Wullt M, Bredberg A, Ryden L, Leandersson K (2015) Systemic monocytic-MDSCs are generated from monocytes and correlate with disease progression in breast cancer patients. PLoS ONE 10(5):e0127028. https://doi.org/10.1371/journal.pone.0127028
Number stages of breast cancer. 2014 http://www.cancerresearchuk.org/about-cancer/breast-cancer/stages-types-grades/number-stages Accessed 2017 June
Bailur JK, Gueckel B, Derhovanessian E, Pawelec G (2015) Presence of circulating Her2-reactive CD8 + T-cells is associated with lower frequencies of myeloid-derived suppressor cells and regulatory T cells, and better survival in older breast cancer patients. Breast Cancer Res 17:34. https://doi.org/10.1186/s13058-015-0541-z
Larbi A, Cabreiro F, Zelba H, Marthandan S, Combet E, Friguet B, Petropoulos I, Barnett Y, Pawelec G (2010) Reduced oxygen tension results in reduced human T cell proliferation and increased intracellular oxidative damage and susceptibility to apoptosis upon activation. Free Radic Biol Med. 48(1):26–34. https://doi.org/10.1016/j.freeradbiomed.2009.09.025
Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R (2010) Immature immunosuppressive CD14 + HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res 70(11):4335–4345. https://doi.org/10.1158/0008-5472.CAN-09-3767
Boniface JD, Poschke I, Mao Y, Kiessling R (2012) Tumor-dependent down-regulation of the zeta-chain in T-cells is detectable in early breast cancer and correlates with immune cell function. Int J Cancer 131(1):129–139. https://doi.org/10.1002/ijc.26355
Poschke I, De Boniface J, Mao Y, Kiessling R (2012) Tumor-induced changes in the phenotype of blood-derived and tumor-associated T cells of early stage breast cancer patients. Int J Cancer 131(7):1611–1620. https://doi.org/10.1002/ijc.27410
Su Z, Ni P, She P, Liu Y, Richard SA, Xu W, Zhu H, Wang J (2017) Bio-HMGB1 from breast cancer contributes to M-MDSC differentiation from bone marrow progenitor cells and facilitates conversion of monocytes into MDSC-like cells. Cancer Immunol Immunother 66(3):391–401. https://doi.org/10.1007/s00262-016-1942-2
Ingold Heppner B, Untch M, Denkert C, Pfitzner BM, Lederer B, Schmitt W, Eidtmann H, Fasching PA, Tesch H, Solbach C, Rezai M, Zahm DM, Holms F, Glados M, Krabisch P, Heck E, Ober A, Lorenz P, Diebold K, Habeck JO, Loibl S (2016) Tumor-infiltrating lymphocytes: a predictive and prognostic biomarker in neoadjuvant-treated HER2-positive breast cancer. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-15-2338
Menard S, Tomasic G, Casalini P, Balsari A, Pilotti S, Cascinelli N, Salvadori B, Colnaghi MI, Rilke F (1997) Lymphoid infiltration as a prognostic variable for early-onset breast carcinomas. Clin Cancer Res. 3(5):817–819
Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, Trebska-McGowan K, Wunderlich JR, Yang JC, Rosenberg SA (2016) Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med 22(4):433–438. https://doi.org/10.1038/nm.4051
Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L (2007) Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 25(18):2546–2553. https://doi.org/10.1200/JCO.2006.08.5829
Mao Y, Sarhan D, Steven A, Seliger B, Kiessling R, Lundqvist A (2014) Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res 20(15):4096–4106. https://doi.org/10.1158/1078-0432.CCR-14-0635
Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, Blosser RL, Tam AJ, Bruno T, Zhang H, Pardoll D, Kim Y (2013) STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 123(4):1580–1589. https://doi.org/10.1172/JCI60083
Gros A, Turcotte S, Wunderlich JR, Ahmadzadeh M, Dudley ME, Rosenberg SA (2012) Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin Cancer Res 18(19):5212–5223. https://doi.org/10.1158/1078-0432.CCR-12-1108
Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB (2010) Immunosuppressive CD14 + HLA-DRlow/- monocytes in prostate cancer. Prostate 70(4):443–455. https://doi.org/10.1002/pros.21078
Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S (2013) Increase in CD14 + HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother 62(8):1421–1430. https://doi.org/10.1007/s00262-013-1447-1
Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, Yancey D, Dahm P, Vieweg J (2008) Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res 14(24):8270–8278. https://doi.org/10.1158/1078-0432.CCR-08-0165
Mao Y, Poschke I, Wennerberg E, Pico de Coana Y, Egyhazi Brage S, Schultz I, Hansson J, Masucci G, Lundqvist A, Kiessling R (2013) Melanoma-educated CD14 + cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res 73(13):3877–3887. https://doi.org/10.1158/0008-5472.CAN-12-4115
Acknowledgements
This work was supported by a grant from the German Research Foundation (DFG Pa 361/22-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical standards
The experiments comply with the current laws of the country in which they were performed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Speigl, L., Burow, H., Bailur, J.K. et al. CD14+ HLA-DR−/low MDSCs are elevated in the periphery of early-stage breast cancer patients and suppress autologous T cell proliferation. Breast Cancer Res Treat 168, 401–411 (2018). https://doi.org/10.1007/s10549-017-4594-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4594-9