Abstract
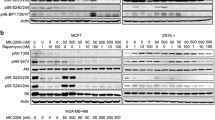
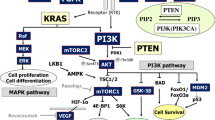
Mammalian target of rapamycin (mTOR) is an attractive target for cancer treatment. While rapamycin and its derivatives (e.g., everolimus) have been shown to inhibit mTOR signaling and cell proliferation in preclinical models of breast cancer, mTOR inhibition has demonstrated variable clinical efficacy with a trend toward better responses in estrogen receptor alpha positive (ERα+) compared to ERα negative (ERα−) tumors. Recently, serum- and glucocorticoid-regulated kinase 1 (SGK1) was identified as a substrate of mTOR kinase activity. Previous studies have alternatively suggested that either mTORC1 or mTORC2 is exclusively required for SGK1’s Ser422 phosphorylation and activation in breast cancer cells. We investigated the effect of rapamycin on the growth of several ERα+ and ERα− breast cancer cell lines and examined differences in the phosphorylation of mTOR substrates (SGK1, p70S6K, and Akt) that might account for the differing sensitivity of these cell lines to rapamycin. We also examined which mTOR complex contributes to SGK1-Ser422 phosphorylation in ERα+ versus ERα− breast cell lines. We then assessed whether inhibiting SGK1 activity added to rapamycin-mediated cell growth inhibition by either using the SGK1 inhibitor GSK650394A or expressing an SGK1 shRNA. We observed sensitivity to rapamycin-mediated growth inhibition and inactivation of insulin-mediated SGK1-Ser422 phosphorylation in ERα+ MCF-7 and T47D cells, but not in ERα− MDA-MB-231 or MCF10A-Myc cells. In addition, either depleting SGK1 with shRNA or inhibiting SGK1 with GSK650394A preferentially sensitized MDA-MB-231 cells to rapamycin. Finally, we found that rapamycin-sensitive SGK1-Ser422 phosphorylation required ERα expression in MCF-7 derived cell lines. Therefore, targeting SGK1 activity may improve the efficacy of rapamycin and its analogs in the treatment of ERα− breast cancer.





Similar content being viewed by others
Abbreviations
- Dex:
-
Dexamethasone
- DMEM:
-
Dulbecco’s modified essential medium
- ERα:
-
Estrogen receptor alpha
- HRP:
-
Horseradish peroxidase
- mTOR:
-
Mammalian target of rapamycin
- shRNA:
-
Short hairpin RNA
- SGK1:
-
Serum and glucocorticoid-regulated protein kinase 1
- PI3 K:
-
phosphoinositide 3-kinase
References
Guertin DA, Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer Cell 12:9–22
Jankiewicz M, Groner B, Desrivieres S (2006) Mammalian target of rapamycin regulates the growth of mammary epithelial cells through the inhibitor of deoxyribonucleic acid binding Id1 and their functional differentiation through Id2. Mol Endocrinol 20:2369–2381
Carraway H, Hidalgo M (2004) New targets for therapy in breast cancer: mammalian target of rapamycin (mTOR) antagonists. Breast Cancer Res 6:219–224
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22:159–168
Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME (2001) Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21:952–965
Mikosz CA, Brickley DR, Sharkey MS, Moran TW, Conzen SD (2001) Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, SGK-1. J Biol Chem 276:16649–16654
Kobayashi T, Cohen P (1999) Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J 339:319–328
Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA (1999) Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J 18:3024–3033
Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM (2008) mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell 30:701–711
Garcia-Martinez JM, Alessi DR (2008) mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J 416:375–385
Chang SB, Miron P, Miron A, Iglehart JD (2007) Rapamycin inhibits proliferation of estrogen-receptor-positive breast cancer cells. J Surg Res 138:37–44
Noh WC, Mondesire WH, Peng J, Jian W, Zhang H, Dong J, Mills GB, Hung MC, Meric-Bernstam F (2004) Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res 10:1013–1023
Witton CJ (2005) Determining sensitivity to rapamycin and its analogues in breast cancer patients. Breast Cancer Res 7:41–42
Liu Q, Chang JW, Wang J, Kang SA, Thoreen CC, Markhard A, Hur W, Zhang J, Sim T, Sabatini DM, Gray NS (2010) Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem 53:7146–7155
Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284:8023–8032
Pew T, Zou M, Brickley DR, Conzen SD (2008) Glucocorticoid (GC)-mediated down-regulation of urokinase plasminogen activator expression via the serum and GC regulated kinase-1/forkhead Box O3a pathway. Endocrinology 149:2637–2645
Belova L, Brickley DR, Ky B, Sharma SK, Conzen SD (2008) Hsp90 regulates the phosphorylation and activity of serum- and glucocorticoid-regulated kinase-1. J Biol Chem 283:18821–18831
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101
Vichai V, Kirtikara K (2006) Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 1:1112–1116
Akcakanat A, Zhang L, Tsavachidis S, Meric-Bernstam F (2009) The rapamycin-regulated gene expression signature determines prognosis for breast cancer. Mol Cancer 8:75
Holz MK, Blenis J (2005) Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem 280:26089–26093
Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM (2006) A pharmacological map of the PI3-K family defines a role for p110[alpha] in insulin signaling. Cell 125:733–747
Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drobak BK, Bjerrum PJ, Christensen SB, Hanley MR (1989) Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions 27:17–23
Hu P, Han Z, Couvillon AD, Exton JH (2004) Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem 279:49420–49429
Sherk AB, Frigo DE, Schnackenberg CG, Bray JD, La** NJ, Trizna W, Hammond M, Patterson JR, Thompson SK, Kazmin D, Norris JD, McDonnell DP (2008) Development of a small-molecule serum- and glucocorticoid-regulated kinase-1 antagonist and its evaluation as a prostate cancer therapeutic. Cancer Res 68:7475–7483
Murray JT, Campbell DG, Morrice N, Auld GC, Shpiro N, Marquez R, Peggie M, Bain J, Bloomberg GB, Grahammer F, Lang F, Wulff P, Kuhl D, Cohen P (2004) Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem J 384:477–488
Inglis SK, Gallacher M, Brown SG, McTavish N, Getty J, Husband EM, Murray JT, Wilson SM (2009) SGK1 activity in Na+ absorbing airway epithelial cells monitored by assaying NDRG1-Thr346/356/366 phosphorylation. Pflugers Arch 457:1287–1301
Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF (2004) The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol 166:213–223
Oesterreich S, Zhang P, Guler RL, Sun X, Curran EM, Welshons WV, Osborne CK, Lee AV (2001) Re-expression of estrogen receptor alpha in estrogen receptor alpha-negative MCF-7 cells restores both estrogen and insulin-like growth factor-mediated signaling and growth. Cancer Res 61:5771–5777
Walker MP, Zhang M, Le TP, Wu P, Laine M, Greene GL (2011) RAC3 is a pro-migratory co-activator of ERalpha. Oncogene 30:1984–1994
Baskin R, Sayeski PP (2011) Angiotensin II mediates cell survival through upregulation and activation of the serum and glucocorticoid inducible kinase 1. Cell Signal 24:435–442
Yamnik RL, Digilova A, Davis DC, Brodt ZN, Murphy CJ, Holz MK (2009) S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J Biol Chem 284:6361–6369
Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, Eiermann W, Hess D, Morant R, Semiglazov V, Borner M, Salzberg M, Ostapenko V, Illiger HJ, Behringer D, Bardy-Bouxin N, Boni J, Kong S, Cincotta M, Moore L (2005) Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol 23:5314–5322
Buckner JC, Forouzesh B, Erlichman C, Hidalgo M, Boni JP, Dukart G, Berkenblit A, Rowinsky EK (2010) Phase I, pharmacokinetic study of temsirolimus administered orally to patients with advanced cancer. Invest New Drugs 28:334–342
Baselga J, Campone M, Sahmoud TPM, Burris H, Rugo H, Noguchi S, Gnant MMP (2011) Everolimus in combination with exemestane for postmenopausal women with advanced breast cancer who are refractory to letrozole or anastrozole: results of the BOLERO-2 phase III trial. J Clin Oncol 29:suppl (abstr e11058)
Bachelot T, Bourgier C, Cropet C, Guastalla J-P, Ferrero J-M, Leger-Falandry C, Soulie P, Eymard J-C, Debled M, Spaeth D, Legouffe E, Delozier T, El Kouri CJC (2010) TAMRAD: a GINECO randomized phase II Trial of everolimus in combination with tamoxifen versus tamoxifen alone in patients (pts) with hormone-receptor positive, HER2 negative metastatic breast cancer (MBC) with prior exposure to aromatase inhibitors (AI). Cancer Res 70:S1–6
Acknowledgments
The authors thank Drs. David Sabatini and Nathanael Gray for supplying Torin1. The authors also thank the members of the Conzen, Greene, and Brady laboratories for useful discussions and critical reading of the manuscript. This study was supported by NIH grants R01CA089208 and R21CA149472 to SDC. TYK was supported by a University of Chicago Biological Sciences Collegiate Division 2011 Summer Research Fellowship.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hall, B.A., Kim, T.Y., Skor, M.N. et al. Serum and glucocorticoid-regulated kinase 1 (SGK1) activation in breast cancer: requirement for mTORC1 activity associates with ER-alpha expression. Breast Cancer Res Treat 135, 469–479 (2012). https://doi.org/10.1007/s10549-012-2161-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2161-y