Abstract
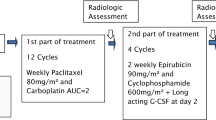
An increased dose-intensity can be achieved by either higher dose of chemotherapy per cycle (dose-escalation) or by shortening the interval between cycles (dose-dense). This multicenter randomized phase II study assessed the efficacy and safety of two different approaches: epirubicin 110 mg/m2 combined with paclitaxel 200 mg/m2 every 21 days and epirubicin 75 mg/m2 combined with paclitaxel 175 mg/m2 every 10 days, both supported with G-CSF. Patients with advanced breast cancer and without prior palliative chemotherapy were scheduled for 6 cycles. Evaluable for response were 101 patients and for toxicity 106 patients. Grade ≥3 toxicities occurred in 39% of patients in the dose-escalated arm and in 29% of the dose-dense arm, mainly febrile neutropenia, thrombocytopenia, neurotoxicity and (asymptomatic) cardiotoxicity. The median delivered cumulative doses for epirubicin/paclitaxel were 656/1194 and 448/1045 mg/m2, treatment durations were 126 and 61 days, and delivered dose intensities were 36/67 and 51/120 mg/m2/week for the dose-escalated and dose-dense arm, respectively. Response rates were 75 and 70%, the progression-free survival 6 and 7 months, respectively. Dose-dense chemotherapy with a lower cumulative dose, a halved treatment time, but a higher dose-intensity may be as effective and safe as dose-escalated chemotherapy. The value of dose-densification over standard scheduled chemotherapy regimes yet needs to be determined.

Similar content being viewed by others
References
A’Hern RP, Smith IE, Ebbs SR (1993) Chemotherapy and survival in advanced breast cancer: the inclusion of doxorubicin in Cooper type regimens. Br J Cancer 67:801–805
Crown J, Diéras V, Kaufmann M, von Minckwitz G et al (2002) Chemotherapy for metastatic breast cancer—report of a European expert panel. Lancet Oncol 3:719–726
Gianni L, Munzone E, Capri G et al (1995) Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer: high antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol 13:2688–2699
Gehl J, Boesgaard M, Paaske T et al (1996) Combined doxorubicin and paclitaxel in advanced breast cancer: effective and cardiotoxic. Ann Oncol 7:687–693
Jain KK, Casper ES, Geller NL et al (1985) A prospective randomized comparison of epirubicin and doxorubicin in patients with advanced breast cancer. J Clin Oncol 3:818–826
Norton L, Simon R (1986) The Norton-Simon hypothesis revisited. Cancer Treat Rep 70:163–169
Norton L (2005) Conceptual and practical implications of breast tissue geometry: toward a more effective, less toxic therapy. Oncologist 10:370–381
Lalisang RI, Wils JA, Nortier HW et al (1997) Comparative study of dose escalation versus interval reduction to obtain dose-intensification of epirubicin and cyclophosphamide with granulocyte colony-stimulating factor in advanced breast cancer. J Clin Oncol 15:1367–1376
Lalisang RI, Voest EE, Wils JA et al (2000) Dose-dense epirubicin and paclitaxel with G-CSF: a study of decreasing intervals in metastatic breast cancer. Br J Cancer 82:1914–1919
Miller AB, Hoogstraten B, Staquet et al (1981) Reporting results of cancer treatment. Cancer 47:207–214
Jassem J, Pienkowski T, Pluzanska A et al (2001) Doxorubicin and paclitaxel versus fluorouracil, doxorubicin, and cyclophosphamide as first-line therapy for women with metastatic breast cancer: final results of a randomized phase III multicenter trial. J Clin Oncol 19:1707–1715
Bontenbal M, Creemers GJ, Braun HJ et al (2005) Phase II to III study comparing doxorubicin and docetaxel with fluorouracil, doxorubicin, and cyclophosphamide as first-line chemotherapy in patients with metastatic breast cancer: results of a Dutch Community Setting Trial for the Clinical Trial Group of the Comprehensive Cancer Centre. J Clin Oncol 23:7081–7088
Biganzoli L, Cufer T, Bruning P et al (2002) Doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first-line chemotherapy in metastatic breast cancer: The European Organization for Research and Treatment of Cancer 10961 Multicenter Phase III Trial. J Clin Oncol 20:3114–3121
Langley RE, Carmichael J, Jones AL et al (2005) Phase III trial of epirubicin plus paclitaxel compared with epirubicin plus cyclophosphamide as first-line chemotherapy for metastatic breast cancer: United Kingdom National Cancer Research Institute trial AB01. J Clin Oncol 23:8322–8330
Nabholtz JM, Falkson C, Campos D et al (2003) Docetaxel and doxorubicin compared with doxorubicin and cyclophosphamide as first-line chemotherapy for metastatic breast cancer: results of a randomized, multicenter, phase III trial. J Clin Oncol 21:968–975
Seidman AD, Berry D, Cirrincione C et al (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 26:1642–1649
Sparano JA, Wang M, Martino S et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–1671
Timmer-Bonte JN, de Mulder PH, Peer PG et al (2005) Timely withdrawal of G-CSF reduces the occurrence of thrombocytopenia during dose-dense chemotherapy. Breast Cancer Res Treat 93:117–123
Citron ML, Berry DA, Cirrincione C et al (2003) Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21:1431–1439
Katsumata N, Yasuda M, Takahashi F et al (2009) Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 374:1331–1338
Sledge GW, Neuberg D, Bernardo P et al (2003) Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193). J Clin Oncol 21:588–592
Alba E, Martin M, Ramos M et al (2004) Multicenter randomized trial comparing sequential with concomitant administration of doxorubicin and docetaxel as first-line treatment of metastatic breast cancer: a Spanish Breast Cancer Research Group (GEICAM-9903) phase III study. J Clin Oncol 22:2587–2593
Conte PF, Guarneri V, Bruzzi P et al (2004) Concomitant versus sequential administration of epirubicin and paclitaxel as first-line therapy in metastatic breast carcinoma: results for the Gruppo Oncologico Nord Ovest randomized trial. Cancer 101:704–712
Biganzoli L, Piccart MJ (1997) The bigger the better? ···or what we know and what we still need to learn about anthracycline dose per course, dose density and cumulative dose in the treatment of breast cancer. Ann Oncol 8:1177–1182
Colozza M, de Azambuja E, Personeni N et al (2007) Achievements in systemic therapies in the pregenomic era in metastatic breast cancer. Oncologist 12:253–270
Acknowledgments
This work was supported in part by grants from Amgen Inc., Pfizer Inc. and Bristol-Myers Squibb Inc.
Conflict of interest
The authors declare that they have no actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within that could inappropriately influence (bias) their work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lalisang, R.I., Erdkamp, F.L.G., Rodenburg, C.J. et al. Epirubicin and paclitaxel with G-CSF support in first line metastatic breast cancer: a randomized phase II study of dose-dense and dose-escalated chemotherapy. Breast Cancer Res Treat 128, 437–445 (2011). https://doi.org/10.1007/s10549-011-1558-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1558-3