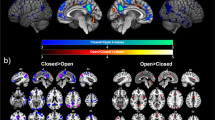
Abstract
This study empirically assessed the strength and duration of short-term effects induced by brain reactions to closing/opening the eyes on a few well-known resting-state networks. We also examined the association between these reactions and subjects’ cortisol levels. A total of 55 young adults underwent 8-min resting-state fMRI (rs-fMRI) scans under 4-min eyes-closed and 4-min eyes-open conditions. Saliva samples were collected from 25 of the 55 subjects before and after the fMRI sessions and assayed for cortisol levels. Our empirical results indicate that when the subjects were relaxed with their eyes closed, the effect of opening the eyes on conventional resting-state networks (e.g., default-mode, frontal-parietal, and saliency networks) lasted for roughly 60-s, during which we observed a short-term increase in activity in rs-fMRI time courses. Moreover, brain reactions to opening the eyes had a pronounced effect on time courses in the temporo-parietal lobes and limbic structures, both of which presented a prolonged decrease in activity. After controlling for demographic factors, we observed a significantly positive correlation between pre-scan cortisol levels and connectivity in the limbic structures under both conditions. Under the eyes-closed condition, the temporo-parietal lobes presented significant connectivity to limbic structures and a significantly positive correlation with pre-scan cortisol levels. Future research on rs-fMRI could consider the eyes-closed condition when probing resting-state connectivity and its neuroendocrine correlates, such as cortisol levels. It also appears that abrupt instructions to open the eyes while the subject is resting quietly with eyes closed could be used to probe brain reactivity to aversive stimuli in the ventral hippocampus and other limbic structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During resting-state functional magnetic resonance imaging (rs-fMRI), subjects remain relaxed with the eyes either closed (EC) or open (EO) without engaging in any task (Fox et al. 2009; Northoff et al. 2010). Several recent studies examining resting states have reported that operational conditions (i.e., eyes-closed, eyes-open, and eyes-open with fixation on an image) greatly affect the generalizability of scientific or clinical findings pertaining to connectivity networks, particularly in the sensory cortex (Andrews-Hanna et al. 2010; Feige et al. 2005; Fox et al. 2005; Kollndorfer et al. 2013; McAvoy et al. 2008; Patriat et al. 2013; Van Dijk et al. 2010; Yeo et al. 2011). In those studies, EC and EO conditions were employed in separate experimental runs, and the first 4 − 10 image volumes of the scanning session were discarded during the data preprocessing phase to allow for T1 equilibrium. Because the auditory instruction was normally brief enough, those discarded image volumes could have also minimized potential confounding effects from brain reactions to closing or opening the eyes. Nevertheless, abrupt auditory instructions could evoke functional states that deviate from previous accounts of resting states and have possible associations with motivation, anxiety and/or neuroendocrine activity (Henning et al. 2006; Yoshida et al. 2019). For example, an abrupt EO instruction could be aversive or novel and invade the consciousness of a subject when being relaxed quietly with eyes closed. Although research into EC/EO has uncovered interesting insights into rs-fMRI connectivity, there is a paucity of empirical data on temporal responses that occur immediately after closing/opening the eyes. There is also a lack of information pertaining to the roles played by neuroendocrine and psychological factors in the regulation of these responses. This study bridges this research gap by empirically assessing the strength and duration of short-term effects induced by brain reactions to closing/opening the eyes on a few well-known resting-state networks (RSNs) and the associations between these reactions and neuroendocrine activity.
Several RSNs have been identified corresponding to basic functions, such as vision, auditory perception, language, episodic memory, executive control and salience detection in the human brain (Damoiseaux et al. 2006; De Luca et al. 2006; Habas et al. 2009; Smith et al. 2009; Yeo et al. 2011). Six highly cited RSNs include the DMN (medial prefrontal cortex, posterior cingulate cortex, precuneus, lateral parietal cortex/angular gyrus, inferior temporal gyrus), fronto-parietal network (FPN; lateral prefrontal and inferior parietal cortices), salience network (SN; the anterior insula and anterior cingulate cortex), meso-paralimbic network (MPN; the amygdala, hippocampal formation, and temporal poles), visual network (VN; the primary and high level visual cortices), and sensorimotor network (SMN; the supplementary motor area, sensorimotor cortex, and secondary somatosensory cortex) (Syan et al. 2017). These networks have been identified primarily based on macroscopically defined regions of interest (ROIs). Some studies using the seed-based approach examined the basal ganglia, thalamus, and hypothalamus when investigating associations between cortisol levels and resting-state connectivity (Peters et al. 2019; Veer et al. 2012). Other studies examined the direct and indirect effects of neuroendocrine concentrations on different brain systems, such as the interaction between limbic structures (hippocampus and amygdala) and the prefrontal cortex (Albert and Newhouse 2019; Noack et al. 2019; Yoshida et al. 2019). The success of those studies has made it clear that any method used to investigate connectivity should be subjected to cross-validation by examining the interplay among connectivity networks, neuroendocrine systems, and psychological factors.
A number of recent studies have shown that movies or other naturalistic stimuli can synchronize brain responses among subjects, resulting in stable spatiotemporal activity patterns with a high degree of similarity (Hasson et al. 2010; Moraczewski et al. 2018; Nastase et al. 2019). In many of those studies, inter-subject correlation (ISC) analysis was used to assess the fMRI time courses that are consistently observed among subjects (Chen et al. 2016; Imhof et al. 2017). In ISC analysis, Pearson correlation is computed between two time-courses in a given ROI, the results of which are then applied to all pairs of subjects. The output values are Fisher Z-transformed, averaged, and finally re-transformed back to a correlation value indicating the average degree of similarity among subjects in terms of time courses pertaining to the given ROI (Schmälzle et al. 2017). Intraclass correlation (ICC), on the other hand, is applicable to cases involving more than a single pair of time-courses, and can be used to obtain a single ISC estimate without having to average multiple pairs of correlation values. Unlike Pearson correlation being scale-free, ICC is sensitive to mean- and variance-differences between time courses. Note that this is not necessarily a flaw, as these differences may reflect clinical severity or developmental changes. Theoretically, the asymptotic properties (e.g., standard error of estimation) of ICC are tractable. This makes the ICC approach a practical alternative to the averaging of correlation values across all pairs of subjects. Note also that ICC analysis is easily implemented within cytoarchitectonically (JuBrain) or macroscopically (AAL) defined ROIs, and remains computationally tractable even when dealing with a fairly large number of subjects.
As with fMRI experiments involving naturalistic stimuli, it is possible to synchronize brain responses among subjects simply by providing the same auditory instructions to keep EC-then-EO (or vice versa) for a specific duration. Individual ROIs in RSNs may exhibit high degrees of ISC when temporal responses induced by brain reactions to auditory instructions are incorporated in data analysis. Because a high degree of synchronicity is likely observed immediately after EC/EO onset and at no other times during resting-state scans, the time courses masked out by the ICC method may still convey information specific to individual subjects, varying as a function of psychological traits and cortisol levels. Within the context of well-known RSNs, our primary objective in the current study was to clarify the strength and duration of short-term effects induced by brain reactions to closing and opening eyes on the DMN, FPN, SN, and MPN, rather than to detail the issue of active versus activated processes under different resting states (Joel et al. 2011; Morcom and Fletcher 2007). We also considered EC/EO effects on the stress-response network (SRN; Lucassen et al. 2014) which is distributed in limbic structures as well as the orbitofrontal cortex. The SRN has been widely discussed in studies on the synthesis of rs-fMRI connectivity and neuroendocrine concentrations. We finally examined the neuroendocrine (cortisol) correlates in these networks to further validate our empirical findings.
Methods
fMRI Data Acquisition and Analysis
Participants and Tasks
A total of 55 right-handed young and healthy adults (27 males; average age 22.93 ± 3.08) were recruited for the rs-fMRI experiment and 36 of them had previous experiences performing tasks in an MRI scanner. The experiment was approved by the Human Participant Research Ethics Committee/Institutional Review Board at Academia Sinica (Taiwan) in accordance with the Declaration of Helsinki, and all subjects gave informed written consent before their enrollment into the study. During the fMRI experiment, each subject received exclusively auditory instructions to close or open the eyes through high-quality MR-compatible insert earphones (Sensimetrics Model S14) worn under hearing protection ear muffs. The 8-min experimental task in this study involved one 4-min cycle under the EC condition followed by one 4-min cycle under the EO condition (herein referred to as EC or EO onset). Under the EC condition, the subject was instructed to rest quietly with eyes closed, and the visual presentation was turned off. Under the EO condition, the subject was instructed to rest quietly with their eyes fixated on a central crosshair, and a red crosshair on a dark background image was presented using an MR-compatible visual stimulation system. Note that the order (EC-EO) was fixed based on the results of a pilot study involving 6 subjects.Footnote 1 The pilot study revealed that this configuration was more relaxing for the subjects. We adopted the 4-min duration with the aim of minimizing motion artifacts, and stabilizing the strength of RSNs (Van Dijk et al. 2010).
Image Acquisition and Preprocessing
The fMRI scan was performed using a 3T MAGNETOM Skyra scanner (Siemens Healthcare, Erlangen, Germany) and a standard 20-channel head-neck coil. The echo planar imaging (EPI) scans were performed with parameters TR/TE = 2000-ms/30-ms, flip angle = 84°, 35 slices, slice thickness = 3.4 mm, FOV = 192 mm, and resolution 3 × 3 × 3.74 mm to cover the whole brain including the cerebellum. There were 120 image volumes collected per condition with a total of two conditions (240 volumes) in each session. In addition, four “dummy” volumes were acquired before the EC/EO paradigm to allow the MR signal to reach steady state, and those volumes were discarded by the scanner. T1-weighted anatomical images were also acquired with the following parameters: TR/TE = 2530-ms/3.30-ms, flip angle = 7°, 192 slices, FOV = 256 mm, and resolution 1 × 1 × 1 mm. The data were preprocessed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm). In line with other rs-fMRI studies, empirical time courses were preprocessed by including motion correction, slice timing, linear detrending, and spatial transformation to the MNI-152 template (Ciric et al. 2017; Elliott et al. 2019; Satterthwaite et al. 2013). Nuisance regressors (e.g., motion parameters) were not included in the preprocessing phase to avoid inflated connectivity values (Caballero-Gaudes and Reynolds 2017; Nalci et al. 2019). Also, bandpass filtering (0.01–0.1 Hz) was not performed in the preprocessing phase for reasons detailed in the Discussion section.
Head motion was corrected by the SPM routine which estimated 6 motion parameters consisting of three translations and three rotations at each time point. The 6 parameters time series were represented by the framewise displacement (FD) and root-mean squared (RMS) displacement values relative to a single reference volume (Satterthwaite et al. 2013). The mean FD relative displacement (MRD) value was computed for each subject. The whole brain signal change was summarized by the derivative of RMS displacement over voxels (DVARS) (Power et al. 2011; Power et al. 2012). All the displacement values were computed by the BRAMILA tools available at (https://users.aalto.fi/~eglerean/bramila.html). There were three subjects whose parameters exceeded 2-mm and \({2}^{\circ }\). Their image volumes were manually divided into two segments. Realignment was performed in accordance with a new reference volume formulated for each segment. The aligned image volumes within individual segments were co-registered individually to the standard MNI template. Table 1 provides a summary of mean and standard deviation of MRD and DVAR values of the 55 subjects along with the subgroup (ten subjects) with the highest motion parameters and the subgroup (ten subjects) with the lowest motion parameters.
The T1-weighted high-resolution image volume was co-registered to the mean of the realigned EPI images and was spatially normalized with voxel size 2 × 2 × 3 mm to the standard MNI-152 space (Note: The anisotropic voxel size was chosen to reflect the resolution of raw data). The signal-to-noise ratio of functional images was enhanced by spatial smoothing using a 4-mm FWHM (full width at half maximum) Gaussian kernel.
Data Analysis
ICC Index
In fMRI applications, two ICC indices have been widely used to assess test–retest reliability between brain activation maps and connectivity networks: ICC(C, M) and ICC(A, M), where M denotes the number of experimental replicates, C refers to consistency, and A refers to agreement (Brandt et al. 2013; Caceres et al. 2009; Fiecas et al. 2013; Kristo et al. 2014; Termenon et al. 2016; Wang et al. 2017; Zanto et al. 2014; Zhu et al. 2014). ICC(C, M) is insensitive to mean differences in intensity among subjects, and therefore gives a slightly higher ISC value when MR images are acquired using different scanners. The empirical values of the indices range from − ∞ to 1 (Lahey et al. 1983). The magnitude of ICC indices is determined by many factors, including the size of M. When computed using M of different sizes, the indices cannot be directly compared with one another unless they are normalized according to standard errors. The standard error of ICC(C, M) for temporally dependent time courses was derived under a stationarity assumption (Kuo et al. 2019). In the sequel, the asymptotic standard error of ICC(A, M) is derived by an analogous approach. In a single voxel, the data forms an n(row)-by-M(column) matrix of image intensities, where the M columns indicate subject-level time courses with n time points in each column. Let S denote an M-by-M column-wise cross-covariance matrix with zero lagged time points among the preprocessed fMRI time courses, and let V denote an n-by-n row-wise covariance matrix. An off-diagonal element in S is computed using a pair of time courses from two subjects as a reflection of their synchronization strength (or similarity). An off-diagonal element in V is computed using a pair of intensity vectors between two points in time as an indication of whether between-subject changes in intensity remain the same at the two time points. The ICC(A, M) or agreement index introduces a mean-sensitive correlation measure by considering the variability in mean intensity values among M subjects and among n time points (McGraw and Wong 1996).
The index has been widely applied in clinical research when investigating test–retest reliability of imaging techniques and connectivity networks (Fiecas et al. 2013; Kristo et al. 2014; Zanto et al. 2014). The index can be expressed as follows:
which is also identical to the ICC(2, M) index in Shrout and Fleiss (1979). A large and positive \(\widehat{\text{A}}\) value indicates that the subject-to-subject variation in their time courses is small; the size of \(\widehat{\text{A}}\) is decreased, on the other hand, if MR images are acquired from different scanners or brain reactivity is unique to individual subjects. In the denominator of (1), Γ denotes the ratio between \(\left({\underline{1}}^{{^{\prime}}}\mathbf{V}\underline{1}/{\text{n}}^{2}\right)\) and \(\left({\underline{1}}^{{^{\prime}}}\mathbf{S}\underline{1}/{\text{M}}^{2}\right)\) where \(\underline{1}\) is the summing vector of order M with the transpose \({\underline{1}}^{^{\prime}}\), and tr(S) denotes the trace of S.
Based on the law of large numbers, the Γ ratio consistently estimates the ratio γ = \({\sigma }_{r}^{2}\)/\({\upsigma }_{\text{c}}^{2}\) as n and M become large. The parameters \({\sigma }_{r}^{2}\) and \({\sigma }_{c}^{2}\) are the mean values of elements in V and S, respectively. The \(\widehat{\text{A}}\) index can be approximated by
In (2), O \(\left(\frac{1}{\text{nM}}\right)\) denotes a remainder term converging to zero with order n times M (Note: The proof can be found in Table A in the Supplementary Materials). The \(\widehat{\text{C}}\) index in (2) denotes the estimate of ICC(C, M) index, which is identical to ICC(3, M) proposed by Shrout and Fleiss (1979). Because \({\varvec{\Gamma}}\) is a positive value, the range of \(\widehat{\text{A}}\) is smaller than that of \(\widehat{\text{C}}\); the \({\varvec{\Gamma}}\) value is affected by the mean differences in intensity among subjects. The variance of \(\widehat{\text{C}}\) has been given in the literature (Browne 1974; Kuo et al. 2019; van Zyl et al. 2000); the asymptotic result in (2) suggests that the variance of \(\widehat{\text{A}}\) can be approximated by
In applications, γ can be evaluated at Γ. The matlab program for computing Var(\(\widehat{\text{C}}\)) is available at (https://github.com/PoChihKuo/icc-neuroimage), which has the following form:
where \({\eta }^{^{\prime}}\) is the derivative of ICC(C, M) with respect to \({\text{vech}}^{\prime}{\varvec{\Sigma}}\) = \(({\text{vech}}{\varvec{\Sigma}})^{\prime},\) and \(\otimes\) is the Kronecker product. \({\varvec{\Sigma}}\) is the population counterpart of S; \({\text{vech}}{\varvec{\Sigma}}\) is the half-vectorization of the matrix Σ; \({\text{K}}_{M}\) is the transformation matrix of order M(M + 1)/2-by-M2, with the identity \({\text{vech}}{\varvec{\Sigma}}={\text{K}}_{M}({\text{vec}}{\varvec{\Sigma}})\), where \({\text{vec}}{\varvec{\Sigma}}\) is the vectorization of the matrix Σ and is of order M2-by-1 and \({\varvec{\Sigma}}\) can be evaluated at S in application. The derivative can be expressed as
where IM is the identity matrix of order M, and \({\text{G}}_{M}\) is the transformation matrix of order M2-by-M(M + 1)/2 with the identity \({\text{vec}}{\varvec{\Sigma}}={\text{G}}_{M}\text{(vech}{\varvec{\Sigma}}\)). The t-value or standardized value for evaluating the empirical agreement index for significance is defined as
The data acquisition provided n = 240 and M = 49 in ISC analysis. A schematic illustration of computing the agreement index, error variance, and \({t}_{\widehat{\text{A}}}\) value in the ICC method can be found in Fig. A-1 in the Supplementary Materials.
Statistical Evaluation of the Stationarity Assumption
The derivation of \(\widehat{A}\) in (2) follows from the law of large numbers (i.e., the sample size n or M is reasonably large). The distribution approximations in (3) and (4) are valid when voxel responses \(\left\{{X}_{k,i}: 1 \le k \le n, 1 \le i \le M\right\}\) across the n image scans have bounded fourth moments and satisfy two basic conditions: (i) strictly stationary along the sequence of image scans, and (ii) ρ-mixing which satisfies the condition that the maximal modulus of lagged correlation values between \({X}_{k,i}\) and \({X}_{k+\upsilon , i}\) converge toward 0, that is, for 1 ≤ k ≤ n–υ and 1 ≤ i ≤ M, or max k, i\(\left|\rho \left( {X}_{k,i},{X}_{k+\upsilon , i}\right)\right|\) → 0 as υ → ∞. Fig. A-2 in the Supplementary Materials provides a graphical proof for the ρ-mixing condition, which shows the bivariate plots of the maximal size of lagged-correlation coefficients against the increasing sequence 10 ≤ υ ≤ 230 given the maximal length n = 240. In the plots, it is clear that the lagged sample correlation coefficients decrease from 0.30 to 0.13 as υ is increasing, suggesting that it can decrease toward 0 when υ increases toward infinity.
It follows from Theorem 1.3 in Bradley (2012) that the stationary elements \({s}_{i,j}\) satisfy asymptotic normality of S, which is the basis for deriving the asymptotic variance of \(\widehat{\text{C}}\) in (4). Several empirical studies have suggested that rs-fMRI time courses tend to be non-stationary (Cole et al. 2010; Lee et al. 2013), which may restrict the use of Var(\(\widehat{\text{A}})\) in (3). When temporal responses induced by brain reactivity to closing and opening the eyes are incorporated into data analysis, however, the problem of non-stationarity might become less relevant. To validate the application of (3) and (4), stationarity condition (i) must be examined. The Kwiatkowski–Phillips–Schmidt–Shin (KPSS) test was applied to individual time courses in all in-brain voxels for evaluating the null hypothesis that time courses are trend-stationary against the alternative of a unit root (Kwiatkowski et al. 1992). In addition, the Augmented Dickey–Fuller (ADF) test was also applied to evaluate the null hypothesis of non-stationarity against the alternative hypothesis of stationarity. A time course can be assumed to be stationary if the KPSS test fails to reject the null hypothesis, and the ADF test rejects the null hypothesis. Among in-brain voxels in the current study, a small portion of time courses (2.02%) were consistently rejected by the KPSS test in at least 70% subjects (4.82% in 60% and 11.79% in 50% subjects). After the weak FDR control, the proportion was reduced to 1.96% in at least 70% subjects (4.64% in 60% and 11.14% in 50% subjects). The rejected time courses were mainly distributed in the subgenual anterior cingulate (Areas s24 and s32), orbitofrontal cortex (Areas Fo1, Fo2, and Fo3), and frontal pole (Fp1, Fp2). None of the time courses was consistently accepted by the ADF test across subjects (i.e., 0.15% time courses was consistently accepted in five subjects). Based on the results, the stationarity condition (i) was found valid in most brain voxels.
ICC Maps
As demonstrated by the stationarity tests, the time courses of in-brain voxels generally satisfied the assumption of stationarity, which partially validated the use of Fourier phase randomization to generate surrogate data for thresholding the empirical \({t}_{\widehat{\text{A}}}\) values when determining statistical significance of these values (Lerner et al. 2011). Familywise Type-I error rate was controlled at α = 0.05 using the false discovery rate (FDR) control procedure (Benjamini and Hochberg 1995; Benjamini and Yekutieli 2001; Genovese et al. 2002; Langers et al. 2007). In the FDR procedure, a sequence of ordered p-values (i.e., the probability of randomly simulated t values in the surrogate distributions greater than or equal to the observed \({t}_{\widehat{\text{A}}}\) values) were compared with the critical value (i/K)[α/C(K)] for the i-th p-value in the ordered sequence to control the FDR at α, where K is the total number of voxels considered and C(K) is a predetermined constant. The choice of constant depends on the joint distribution of p-values in the sequence. It has previously been established that FDR control may increase the false-positive or false-negative rate depending on the overall proportion of voxels that are truly synchronized among subjects (Chumbley et al. 2010). In the current study, C(K) = \({\sum }_{i=1}^{K}{i}^{-1}\) was selected for a weak control of the false positive rate (Genovese et al. 2002). The ICC maps were constructed by identifying the supra-threshold voxels with the \({t}_{\widehat{\text{A}}}\) values exceeding the FDR critical values. The supra-threshold maps were co-registered to the JuBrain Cytoarchitectonic Atlas available at (http://www.fz-juelich.de/inm/inm-7/tools) for the topographical definition of area-specific effects (Eickhoff et al. 2005).
Types of Short-Term Effects
Table 2 presents a list of cytoarchitectonic areas involved in the DMN, MPN, FPN and SN and their corresponding Brodmann nomenclature. We annotated brain areas linked to the DMN in a meta-analysis of nine Positron Emission Tomography (PET) studies (132 subjects) by Shulman et al. (1997). After re-analyzing the PET data, Buckner et al. (2005) identified DMN areas that were more active during passive tasks than during active tasks (See Figure 2 in Buckner et al. (2008)). Zilles (2017) included anatomical correspondence between the DMN in Buckner et al. (2008) and cytoarchitectonic areas in JuBrain. In the current study, we also considered cortisol levels, and therefore examined brain structures associated with the SRN (Cerqueira et al. 2008; Lucassen et al. 2014), including the orbitofrontal cortex, hippocampus, entorhinal cortex, amygdala, nucleus accumbens, basal forebrain, and subgenual anterior cingulate cortex. Brain areas unavailable in JuBrain were defined using the Neuromorphometrics Inc. Atlas (http://neuromorphometrics.com) and MRIcro software (http://www.mricro.com) in support of network analysis.
For each ROI (e.g., Area 25 in the sACC) in Table 2, we identified supra-threshold voxels with significant \({t}_{\widehat{A}}\) values. We initially classified the corresponding mean time courses across subjects within each target ROI using iterative K-means clustering and the Silhouette statistics. This was meant to ensure that time courses were homogeneous within each ROI. In cases where an ROI contained two types of time courses (e.g., anterior insula), the ROI was further partitioned into two sub-ROIs. Following an initial examination, the time courses were averaged within each ROI or sub-ROI at the subject level for use in deriving connectivity values. Mean time courses at the subject-level were pooled and averaged at the group level (i.e., one mean time course in each ROI or sub-ROI) for classification into homogeneous networks using iterative K-means clustering (i.e., This procedure was to identify various types of short-term effects). The optimal size for K (number of networks) was determined using the Silhouette statistic. Interested readers may refer to p.88 in Kaufman and Rousseeuw (2009) for a qualitative interpretation of the Silhouette statistic.
Correlation values were computed using subject-level mean time courses between each pair of ROIs within a given network or cluster (Betti et al. 2013; O'Neil et al. 2014; Wang et al. 2007) and between two networks. Fisher's Z transformation was used to enhance the Gaussianity of correlation values. We identified 55 Fisher Z-transformed correlation values between each pair of ROIs (each connectivity value), the sample mean of which was evaluated for statistical significance using the Student’s t-test (Betti et al. 2013; O'Neil et al. 2014; Wang et al. 2007) with the weak FDR control of family-wise error rates. To facilitate visualization, connectivity networks were reconstructed using the BrainNet Viewer (** regions in human posterior parietal cortex. Neuroimage 191:234–242" href="/article/10.1007/s10548-022-00897-x#ref-CR92" id="ref-link-section-d20627697e6477">2019). The SPC is heavily interconnected with the dorsal premotor cortex (Wise et al. 1997), which is important in response selection, stimulus–response association, and response preparation (Passingham 1993). Function in the left AIns is specific to the perception of visual emotional stimuli (Caria et al. 2010). As mentioned previously, Area vHC initiated the orienting response to novel (aversive) stimuli, thereby engaging motor immobility as a parasympathetic brake on the otherwise active motor system to sustain goal-directed behavior. To summarize, it appears that auditory instructions could decrease activity in brain areas that were functionally unrelated to controlling eye-movement. Moreover, decreased activity in Area vHC and the motor system might be an indication of a suppressed fight-or-flight response with the aim of sustaining the on-going resting-state.
Stress-Response Network
One recent review in which animal models were combined with diffusion imaging tractography proposed a revision to the conventional limbic model, which treats the anterior temporal cortex (AM and TP) and OFC as a network functionally associated with behavioral inhibition (Catani et al. 2013). In the theory of behavioral inhibition proposed by Gary and Naughton, the septal nucleus (Note: Nucleus Ch1 in the BF is the medial septal nucleus) and hippocampus play critical roles in monitoring approach-avoidance conflicts. In that schema, activation of the HC constitutes non-anxious rumination, activation of the AM constitutes pure fear, and activation of both constitutes anxiety (McNaughton and Gray 2000). Thus, the AM is strongly engaged in increasing arousal when subjects encounter fear potentiated startle. Neuroimaging and post-mortem histological studies on patients suffering from chronic anxiety and depression have also revealed stress-induced changes in brain structures, including the OFC, sACC, HT, insular cortex, and EnC (Cerqueira et al. 2008; Lucassen et al. 2014). The insular cortex is commonly included as part of the stress-response system (especially the anterior insula) (Bogdan et al. 2016; Hofman and Falk 2012; Nieuwenhuys 2011); however, time courses obtained from the anterior insula in this study are suggestive of two processes: One associated with the TPN and the other associated with the TNN. Generally, brain areas in the SRN overlap considerably with the MPN, which deals with information related to emotion processing and introspection.
In the current study, the SRN exhibited a prolonged decrease in activity following the eyes-closed instruction, and a short-term decrease succeeded by a long-term increase in activity following the eyes-open instruction. Limbic deactivation has been interpreted as the suppression of unwanted emotional reactions that are aroused by tasks (Dagher et al. 2009; Dedovic et al. 2009; Lederbogen et al. 2011; Pruessner et al. 2008; Soliman et al. 2011), or conversely as full relaxation responses under resting states (Kalyani et al. 2011; Shetkar et al. 2019). As mentioned previously, a short-term decrease in the vHC was an indication that the subject was under parasympathetic control and free from approach-avoidance conflicts. Thus, it would be reasonable to hypothesize that while the subjects were relaxed under the EC condition, the SRN played a role in monitoring the external world using sensory information. A prolonged decrease in activity when the subject became accustomed to the external environment might be an indication of fMRI repetition suppression (Barron et al. 2016; Bunzeck and Thiel 2016; Grill-Spector et al. 2006). In other words, the prolonged decrease in activity might be an indication of reduced connectivity between the network and cortical structures (Barron et al. 2016; Kuo et al. 2019). Researchers have previously demonstrated that goal-directed eye movements can activate a dorsal fronto-parietal network (i.e., TPN) and transiently deactivate the amygdala (i.e., SRN) via a ventromedial prefrontal pathway to enable the cognitive regulation of emotions (de Voogd et al. 2018). These findings are consistent with our observation of time courses within the SRN under the EO condition. To summarize, the SRN presented reduced connectivity to cortical structures under the EC condition. It is possible that when the novel (aversive) stimulus was non-threatening, the observed short-term decrease succeeded by a long-term increase in activity under the EO condition was mediated by either the ventromedial prefrontal pathway or Area vHC (Patrick et al. 2019).
Pre-scan and Post-scan Cortisol Levels
Cortisol is a catabolic hormone in normal life and an adaptation hormone in response to stressful situations (Coenen and Flik 2017). Cortisol acts on low-affinity glucocorticoid receptors (GRs) as well as high-affinity mineralocorticoid receptors (MRs). GRs are widely distributed throughout the brain with high concentrations in the hypothalamic corticotrophin-releasing-hormone neurons and pituitary corticotropes and, by contrast, the distribution of MRs is highly restricted (Andersen et al. 2013; de Kloet and Joëls 2020; Jelić et al. 2005). Both GRs and MRs are highly expressed in limbic structures, such as the hippocampus, amygdala, and brain areas associated with fear and anxiety (De Kloet et al. 2018; Deuter et al. 2019). In the current study, average cortisol levels in saliva collected prior to the fMRI scanning sessions were significantly lower than in saliva collected after scanning. Connectivity values in the three networks were also more strongly associated with pre-cortisol levels than with post-cortisol levels. As mentioned, subjects were passively exposed to 20-min of natural sound stimulation (human voices and animal vocalizations) and speech (same sex-marriage controversy) following the completion of the resting-state scan. The STAI-Trait and SQ-ISMA scores were more strongly correlated with post-cortisol levels than with pre-cortisol levels. In other words, trait-anxiety or stress scores could be predictive of post-cortisol levels.
The results in Tables 4 and 5 show that after controlling for demographic factors, connectivity within the SRN was significantly positively correlated with pre-cortisol levels. Inter-hemispheric connectivity within the SRN and TNN was also significantly correlated with pre-cortisol levels. These results are in good agreement with the high concentrations of GRs and MRs in limbic structures. As mentioned previously, ROIs in the SRN were stable loci for probing cortisol activity, despite their sensitivity to the EO instruction while the subjects were resting quietly. The strong connection between the TNN and SRN under the EC condition might account for the significant correlation between connectivity in the TNN and pre-cortisol levels. It is also possible that under the EC condition, connectivity within and between the SRN and TNN could serve as a predictor of post-cortisol levels. The EC protocol is generally well-suited to research linking resting-state connectivity and GR/MR concentration in limbic structures, considering the weakness of brain reactions to the anticipated EC instruction. Our empirical data collected from one cycle of EC-EO resting state scanning did not support the hypothesis that decreased activity in the ventral hippocampus is regulated by cortisol activity, as indicated by the fact that the TNN was unaffected by pre-cortisol levels under the EO condition. Moreover, the correlation values listed in Tables 4, 5, C and D do not necessarily provide evidence supporting the role played by pre-cortisol levels in regulating EC/EO effects. As mentioned, subject-level mean time courses showed a high degree of synchronicity after EC/EO onset and at no other times. This would be a reason that we found a significant correlation between connectivity values and pre-cortisol levels within the SRN. Therefore, the ICC method provided ample information specific to individual subjects when searching for synchronized brain reactions to EC/EO instructions.
Remarks
Rs-fMRI studies have previously used 0.01 Hz (or 0.008 Hz) as a standard cutoff to remove low frequency effects such as slow drifts in the fMRI signal (Wang 2021). Physiological noise from respiratory and cardiac functions is concentrated at higher frequencies (> 0.1 Hz) (Davey et al. 2013; Mascali et al. 2021). Thus, RSNs of interest are generally associated with frequencies in the range of 0.01–0.1 Hz. Fig. H in the Supplementary Materials plots supra-threshold time courses of the 49 subjects (receiving EC-EO instructions) after either high-pass (i.e., > 0.01 Hz) or bandpass (0.01–0.1 Hz) filtering. Note that the supra-threshold time courses were extracted using the ICC method based on pre-processed data that had not undergone filtering. In a comparison of Figs. 3, 4, 5 and H, it appears that the TPN was less affected by data filtering than were the TNN and SRN. Figures H-(b), H-(c), H-(bʹ) and H-(cʹ) further suggest that ROIs in the TNN and SRN after data filtering could be grouped into one cluster, such that activity in the ventral hippocampus and other motor areas would be indifferentiable from that in the SRN. The main hypothesis generated from our findings was the approach-avoidance conflicts which distinguished activity in the hippocampus (the transition negative network; TNN) from that in limbic structures (the stress-response network; SRN). The two networks were highly associated with each other under the EC condition, but disconnected under the EO condition. As mentioned, the SRN is significantly associated with pre-cortisol levels under both EC and EO conditions, but the TNN is significantly associated with pre-cortisol levels only under the EC condition. The filtering procedure (especially high-pass filtering) in the pre-processing stage could affect the scientific interpretation of TNN and SRN.
Changes in heart-rate and respiration could have confounding effects on rs-fMRI time courses. Because electrocardiograms (ECGs) and breathing patterns were not collected during resting-state scans, it is difficult to provide a concise assessment of these effects. However, high-pass and bandpass filtering provided similar results as indicated in Fig. H. It appears that heart-rate and respiration might not necessarily have a serious confounding effect on the analysis of brain reactions to closing/opening the eyes. Head motion after EC/EO onset could also affect the assessment of synchronicity in the ICC method. Values of FD and DVARS across the time scale are presented in Fig. F in the Supplementary Materials for the 49 subjects receiving EC-EO instructions. These values were computed using realigned image volumes within individual subjects before the stages of removing trends and normalization to the MNI template. There were two subjects who did show larger FD values after EC/EO onset compared with other subjects. In Fig. F, the large mean values after EC/EO onset (especially after EC onset) mainly reflect head motion of these two highlighted subjects (e.g., See blue and red curves). Because the ICC method considers synchronicity across all subjects, we assume that head motion of a few subjects after EC/EO onset had minor effects on interpretation of the three networks.
Limitations
The order of EC and EO instructions had minimum effects on the TPN, but had significant effects on the TNN and SRN according to brain reactions to auditory instructions in the 6 pilot subjects. However, the order of instructions did not show significant effects on the assessment of correlation between connectivity and cortisol levels. Our experiment was limited to one cycle of the EC-EO transition, and findings in the Results section could partially reflect a variety of diverse processes such as listening to instructions, opening the eyes, and novelty responses. The experimental design did not allow to disentangle the specific contributions of these processes to the observed data. A further study could adopt multiple cycles of the EC-EO transitions to investigate effects from different processes (Feige et al. 2005). Budget considerations limited us to 25 assays for cortisol levels (including cortisol levels in the 6 pilot subjects). Given the small sample size in the current study, we were unable to provide a reliable test on the hypothesis of approach-avoidance conflicts as regulated by Area vHC (or alternatively by a ventromedial prefrontal pathway).
Conclusion
The study is the first attempt to connect cytoarchitectonically defined brain structures to brain reactivity patterns associated with closing and opening the eyes under resting-state conditions. We recommend that research based on rs-fMRI adopts the EC condition when probing resting-state connectivity and its neuroendocrine correlates. We also suggest that abrupt instructions to open the eyes (while subjects are resting quietly with EC) could be used as a probe to characterize brain reactivity to aversive stimuli in the ventral hippocampus and other limbic structures. Finally, we encourage further work in this direction, particularly from a clinical perspective.
Data Availability
FMRI raw data are available upon request to the corresponding author.
Notes
Among the 55 subjects, 49 of them received the EC-EO instructions and the 6 pilot subjects received the EO-EC instructions. Suprathreshold voxels masked out by the ICC method were found by the evaluation of synchronicity among the 49 subjects (who followed the same order of instructions). The spatial locations of these voxels were applied to extract time courses of the 6 pilot subjects. The evaluation of statistical assumptions underlying the ICC method and the connectivity study were applied to the 55 subjects. The pilot subjects were interviewed on their experiences of the experimental procedures after fMRI scanning.
References
Ajayi IE et al (2018) Hippocampal modulation of cardiorespiratory function. J Respir Physiol Neurobiol 252:18–27
Albert KM, Newhouse PA (2019) Estrogen, stress, and depression: cognitive and biological interactions. Ann Rev Clin Psychol 15:399–423
Amunts K et al (1999) Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412:319–341
Andersen M, Vinther F, Ottesen JT (2013) Mathematical modeling of the hypothalamic–pituitary–adrenal gland (HPA) axis, including hippocampal mechanisms. Math Biosci 246:122–138
Andrews-Hanna JR et al (2010) Functional-anatomic fractionation of the brain’s default network. Neuron 65:550–562
Ardekani BA et al (2002) Functional magnetic resonance imaging of brain activity in the visual oddball task. Cogn Brain Res 14:347–356
Argaman Y et al (2020) The endogenous analgesia signature in the resting brain of healthy adults and migraineurs. J Pain 21:905–918
Baltaretu BR et al (2020) Parietal cortex integrates object orientation and saccade signals to update grasp plans. J Neurosci 40(23):4525–4535
Barron HC, Garvert MM, Behrens TE (2016) Repetition suppression: a means to index neural representations using BOLD? Philos. Trans R Soc Lond B Biol Sci 371:20150355
Battaglia-Mayer A et al (2003) Multiple levels of representation of reaching in the parieto-frontal network. Cereb Cortex 13:1009–1022
Battaglia-Mayer A, Caminiti R (2018) Parieto-frontal networks for eye–hand coordination and movements. In: Vallar G, Coslett HB (eds) The parietal lobe, vol 151. Elsevier, Amsterdam, pp 499–524
Becker JT et al (1999) A comment on the functional localization of the phonological storage subsystem of working memory. Brain Cogn 41:27–38
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57:289–300
Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188
Betti V et al (2013) Natural scenes viewing alters the dynamics of functional connectivity in the human brain. Neuron 79:782–797
Bludau S et al (2014) Cytoarchitecture, probability maps and functions of the human frontal pole. Neuroimage 93(Pt 2):260–275
Bogdan R et al (2016) Genetic moderation of stress effects on corticolimbic circuitry. Neuropsychopharmacol 41:275–296
Bradley RC (2012) On the behavior of the covariance matrices in a multivariate central limit theorem under some mixing conditions. Illinois J Math 56:677–704
Brandt DJ et al (2013) Test-retest reliability of fMRI brain activity during memory encoding. Front Psychiatry 4:163
Browne MW (1974) Generalized least squares estimators in the analysis of covariance structures. South African Statist J 8:1–24
Buckner RL et al (2005) Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25:7709–7717
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38
Bunzeck N, Thiel C (2016) Neurochemical modulation of repetition suppression and novelty signals in the human brain. Cortex 80:161–173
Byrne DG, Davenport SC, Mazanov J (2007) Profiles of adolescent stress: the development of the adolescent stress questionnaire (ASQ). J Adolesc 30:393–416
Caballero-Gaudes C, Reynolds RC (2017) Methods for cleaning the BOLD fMRI signal. Neuroimage 154:128–149
Caceres A et al (2009) Measuring fMRI reliability with the intra-class correlation coefficient. Neuroimage 45:758–768
Campana G et al (2007) Left frontal eye field remembers “where” but not “what.” Neuropsychologia 45:2340–2345
Caria A et al (2010) Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biol Psychiat 68:425–432
Carrasco M (2011) Visual attention: the past 25 years. Vis Res 51:1484–1525
Caspers S et al (2008) The human inferior parietal lobule in stereotaxic space. Brain Struct Funct 212:481–495
Catani M, Dell’Acqua F, De Schotten MT (2013) A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev 37:1724–1737
Cerqueira JJ, Almeida OF, Sousa N (2008) The stressed prefrontal cortex. Left? Right! Brain Behav Immun 22:630–638
Chambers CD et al (2004) Fast and slow parietal pathways mediate spatial attention. Nat Neurosci 7:217–218
Chen G et al (2016) Untangling the relatedness among correlations, part I: nonparametric approaches to inter-subject correlation analysis at the group level. Neuroimage 142:248–259
Chumbley J et al (2010) Topological FDR for neuroimaging. Neuroimage 49:3057–3064
Ciric R et al (2017) Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage 154:174–187
Clarke S et al (2000) Auditory agnosia and auditory spatial deficits following left hemispheric lesions: evidence for distinct processing pathways. Neuropsychologia 38:797–807
Coenen A, Flik G (2017) Effects of stress on behavior and sleep. Cortisol: a stress hormone and a wake hormone? Proc Shevchenko Sci Soc 49:53–57
Cole DM, Smith SM, Beckmann CF (2010) Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci 4:8
Dagher A et al (2009) An acute psychosocial stress enhances the neural response to smoking cues. Brain Res 1293:40–48
Damasio H et al (2001) Neural correlates of naming actions and of naming spatial relations. Neuroimage 13:1053–1064
Damoiseaux JS et al (2006) Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853
Davey CE et al (2013) Filtering induces correlation in fMRI resting state data. Neuroimage 64:728–740
De Kloet E et al (2018) Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front Neuroendocrinol 49:124–145
de Kloet ER, Joëls M (2020) Mineralocorticoid receptors and glucocorticoid receptors in HPA stress responses during co** and adaptation. In: de Kloet ER (ed) Oxford research encyclopedia of neuroscience. Oxford University Press, Oxford
De Luca M et al (2006) fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage 29:1359–1367
de Voogd LD et al (2018) Eye-movement intervention enhances extinction via amygdala deactivation. J Neurosci 38:8694–8706
Dedovic K et al (2009) The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage 47:864–871
Deuter CE et al (2019) Influence of glucocorticoid and mineralocorticoid receptor stimulation on task switching. J Horm Behav 109:18–24
Eickhoff SB et al (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335
Elliott ML et al (2019) General functional connectivity: Shared features of resting-state and task fMRI drive reliable and heritable individual differences in functional brain networks. Neuroimage 189:516–532
Faillenot I et al (1997) Visual pathways for object-oriented action and object recognition: functional anatomy with PET. Cereb Cortex 7:77–85
Feige B et al (2005) Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. J Neurophysiol 93:2864–2872
Fiecas M et al (2013) Quantifying temporal correlations: a test–retest evaluation of functional connectivity in resting-state fMRI. Neuroimage 65:231–241
Fox MD et al (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678
Fox MD et al (2009) The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101:3270–3283
Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870–878
Gonzalez CC, Billington J, Burke MR (2016) The involvement of the fronto-parietal brain network in oculomotor sequence learning using fMRI. Neuropsychologia 87:1–11
Grill-Spector K, Henson R, Martin A (2006) Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci 10:14–23
Guyenet PG (2006) The sympathetic control of blood pressure. Nat Rev Neurosci 7:335–346
Habas C et al (2009) Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29:8586–8594
Hagenaars MA, Oitzl M, Roelofs K (2014) Updating freeze: aligning animal and human research. Neurosci Biobehav Rev 47:165–176
Hanson JL et al (2019) Resting state coupling between the amygdala and ventromedial prefrontal cortex is related to household income in childhood and indexes future psychological vulnerability to stress. J Dev Psychopathol 31:1053–1066
Hasson U, Malach R, Heeger DJ (2010) Reliability of cortical activity during natural stimulation. Trends Cogn Sci 14:40–48
Hebart MN, Hesselmann GJJON (2012) What visual information is processed in the human dorsal stream? J Neurosci 32:8107–8109
Henning S, Merboldt KD, Frahm J (2006) Task- and EEG-correlated analyses of BOLD MRI responses to eyes opening and closing. Brain Res 1073–1074:359–364
Henssen A et al (2016) Cytoarchitecture and probability maps of the human medial orbitofrontal cortex. Cortex 75:87–112
Hirnstein M et al (2011) TMS over the left angular gyrus impairs the ability to discriminate left from right. Neuropsychologia 49:29–33
Hofman M, Falk D (2012) The insular cortex: a review. In: Hofman M (ed) Evolution of the primate brain: from neuron to behavior. Elsevier, Amsterda, pp 123–163
Hutchison RM et al (2012) Functional connectivity of the frontal eye fields in humans and macaque monkeys investigated with resting-state fMRI. J Neurophysiol 107:2463–2474
Imhof MA et al (2017) How real-life health messages engage our brains: Shared processing of effective anti-alcohol videos. Soc Cogn Affect Neurosci 12:1188–1196
Ischebeck A et al (2021) Target processing in overt serial visual search involves the dorsal attention network: a fixation-based event-related fMRI study. Neuropsychologia 153:107763
Jelić S, Čupić Ž, Kolar-Anić L (2005) Mathematical modeling of the hypothalamic–pituitary–adrenal system activity. Math Biosci 197:173–187
Joel SE et al (2011) On the relationship between seed-based and ICA-based measures of functional connectivity. Magn Reson Med 66:644–657
Kalyani BG et al (2011) Neurohemodynamic correlates of ‘OM’chanting: a pilot functional magnetic resonance imaging study. Intl J Yoga 4:3
Kaufman L, Rousseeuw PJ (2009) Finding groups in data: an introduction to cluster analysis, vol 344. Wiley, New York
Klaus J, Hartwigsen GJHBM (2019) Dissociating semantic and phonological contributions of the left inferior frontal gyrus to language production. Hum Brain Mapp 40:3279–3287
Kollndorfer K et al (2013) A systematic investigation of the invariance of resting-state network patterns: is resting-state fMRI ready for pre-surgical planning? Front Hum Neurosci 7:95
Kraus KS, Canlon B (2012) Neuronal connectivity and interactions between the auditory and limbic systems. Effects of noise and tinnitus. Hear Res 288:34–46
Kristo G et al (2014) Task and task-free FMRI reproducibility comparison for motor network identification. Hum Brain Mapp 35:340–352
Kuntze LB et al (2016) Ventral hippocampus modulates bradycardic response to peripheral chemoreflex activation in awake rats. Exp Physiol 101:482–493
Kuo P-C et al (2019) Brain dynamics and connectivity networks under natural auditory stimulation. Neuroimage 202:116042
Kwiatkowski D et al (1992) Testing the null hypothesis of stationarity against the alternative of a unit root. J Econometrics 54:159–178
Lahey MA, Downey RG, Saal FE (1983) Intraclass correlations: there’s more there than meets the eye. Psychol Bull 93:586–595
Lane AR et al (2013) Near and far space: understanding the neural mechanisms of spatial attention. Hum Brain Mapp 34:356–366
Langers DR, Jansen JF, Backes WH (2007) Enhanced signal detection in neuroimaging by means of regional control of the global false discovery rate. Neuroimage 38:43–56
Laurienti PJ et al (2002) Deactivation of sensory-specific cortex by cross-modal stimuli. J Cogn Neurosci 14:420–429
Lederbogen F et al (2011) City living and urban upbringing affect neural social stress processing in humans. Nature 474:498
Lee H-L et al (2013) Tracking dynamic resting-state networks at higher frequencies using MR-encephalography. Neuroimage 65:216–222
Lerner Y et al (2011) Topographic map** of a hierarchy of temporal receptive windows using a narrated story. J Neurosci 31:2906–2915
Levenstein S et al (1993) Development of the perceived stress questionnaire: a new tool for psychosomatic research. J Psychosom Res 37:19–32
Lucassen PJ et al (2014) Neuropathology of stress. Acta Neuropathol 127:109–135
Magri C et al (2019) Directional tuning for eye and arm movements in overlap** regions in human posterior parietal cortex. Neuroimage 191:234–242
Mascali D et al (2021) Evaluation of denoising strategies for task-based functional connectivity: equalizing residual motion artefacts between rest and cognitively demanding tasks. Hum Brain Mapp 42:1805–1828
Mayhew SD et al (2013) Spontaneous EEG alpha oscillation interacts with positive and negative BOLD responses in the visual–auditory cortices and default-mode network. Neuroimage 76:362–372
McAvoy M et al (2008) Resting states affect spontaneous BOLD oscillations in sensory and paralimbic cortex. J Neurophysiol 100:922–931
McGraw KO, Wong SP (1996) Forming inferences about some intraclass correlation coefficients. Psychol Methods 1:30–46
McNaughton N, Gray JA (2000) Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disord 61:161–176
Moraczewski D, Chen G, Redcay E (2018) Inter-subject synchrony as an index of functional specialization in early childhood. Sci Rep 8:1–12
Morcom AM, Fletcher PCJN (2007) Does the brain have a baseline? Why we should be resisting a rest. Neuroimage 37:1073–1082
Morgenroth E et al (2020) Using connectivity-based real-time fMRI neurofeedback to modulate attentional and resting state networks in people with high trait anxiety. Neuroimage 25:102191
Mort DJ et al (2003) Differential cortical activation during voluntary and reflexive saccades in man. Neuroimage 18:231–246
Munoz-Lopez M, MohedanoMoriano A, Insausti R (2010) Anatomical pathways for auditory memory in primates. Front Neuroanat 4:129
Nalci A, Rao BD, Liu TT (2019) Nuisance effects and the limitations of nuisance regression in dynamic functional connectivity fMRI. Neuroimage 184:1005–1031
Nastase SA et al (2019) Measuring shared responses across subjects using intersubject correlation. Oxford University Press, Oxford
Neggers SF et al (2012) A functional and structural investigation of the human fronto-basal volitional saccade network. PLoS ONE 7:e29517
Nieuwenhuys R (2011) The insular cortex: a review. Prog Brain Res 195:123–163
Noack H et al (2019) Imaging stress: an overview of stress induction methods in the MR scanner. J Neural Transm 126:1187–1202
Northoff G, Duncan NW, Hayes DJJPIN (2010) The brain and its resting state activity—experimental and methodological implications. Prog Neurobiol 92:593–600
O’Neil EB et al (2014) Resting-state fMRI reveals functional connectivity between face-selective perirhinal cortex and the fusiform face area related to face inversion. Neuroimage 92:349–355
Olson EA et al (2019) Regional prefrontal resting-state functional connectivity in posttraumatic stress disorder. Biol Psychol 4:390–398
Palomero-Gallagher N et al (2009) Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum Brain Mapp 30:2336–2355
Palomero-Gallagher N et al (2020) Multimodal map** and analysis of the cyto-and receptorarchitecture of the human hippocampus. Brain Struct Funct 225:881–907
Passingham RE (1993) The frontal lobes and voluntary action. Oxford University Press, Oxford
Patriat R et al (2013) The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. Neuroimage 78:463–473
Patrick F et al (2019) Brain activation during human defensive behaviour: a systematic review and preliminary meta-analysis. Neurosci Biobehav Rev 98:71–84
Peters AT et al (2019) Pre-scan cortisol is differentially associated with enhanced connectivity to the cognitive control network in young adults with a history of depression. Psychoneuroendocrinology 104:219–227
Pierrot-Deseilligny C et al (2003) Cortical control of ocular saccades in humans: a model for motricity. Prog Brain Res 142:3–17
Pierrot-Deseilligny C, Milea D, Muri RM (2004) Eye movement control by the cerebral cortex. Curr Opin Neurol 17:17–25
Power JD et al (2011) Functional network organization of the human brain. Neuron 72:665–678
Power JD et al (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154
Pruessner JC et al (2008) Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry 63:234–240
Rizzolatti G, Matelli MJEBR (2003) Two different streams form the dorsal visual system: anatomy and functions. Exp Brain Res 153:146–157
Roelofs K (2017) Freeze for action: neurobiological mechanisms in animal and human freezing. Philos Trans Royal Soc B 372:20160206
Satterthwaite TD et al (2013) An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64:240–256
Schaal NK, Pollok B, Banissy MJ (2017) Hemispheric differences between left and right supramarginal gyrus for pitch and rhythm memory. Sci Rep 7:1–6
Scheperjans F et al (2008) Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex 18:2141–2157
Schmälzle R, et al (2017) Reliability of fMRI time series: Similarity of neural processing during movie viewing. bioRxiv preprint first posted online Jul. 3, 2017. https://doi.org/10.1101/158188.
Seghier ML (2013) The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19:43–61
Shek DT (1993) The Chinese version of the state-trait anxiety inventory: its relationship to different measures of psychological well-being. J Clin Psychol 49:349–358
Shetkar RM et al (2019) Association between cyclic meditation and creative cognition: optimizing connectivity between the frontal and parietal lobes. Intl J Yoga 12:29
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428
Shulman GL et al (1997) Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 9:648–663
Singh-Curry V, Husain M (2009) The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia 47:1434–1448
Smith SM et al (2009) Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045
Soliman A et al (2011) Limbic response to psychosocial stress in schizotypy: a functional magnetic resonance imaging study. Schizophr Res 131:184–191
Spielberger CD (2010) State-trait anxiety inventory. In: Weiner IB (ed) The Corsini encyclopedia of psychology. Wiley, Hoboken, pp 1–1
Syan SK et al (2017) Influence of endogenous estradiol, progesterone, allopregnanolone, and dehydroepiandrosterone sulfate on brain resting state functional connectivity across the menstrual cycle. Fertil Steril 107:1246–1255
Termenon M et al (2016) Reliability of graph analysis of resting state fMRI using test-retest dataset from the human connectome project. Neuroimage 142:172–187
Torrisi S et al (2019) Resting-state connectivity of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in clinical anxiety. J Psychiatry Neurosci 44:313
Tyc VL et al (1995) Children’s distress during magnetic resonance imaging procedures. Child Health Care 24:5–19
Ungerleider LG, Mishkin M (1982) Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW (eds) Analysis of visual behavior. MIT Press, Cambridge, pp 549–586
Van Dijk KR et al (2010) Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103:297–321
van Zyl JM, Neudecker H, Nel DG (2000) On the distribution of the maximum likelihood estimator of Cronbach’s alpha. Psychometrika 65:271–280
Veer IM et al (2012) Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology 37:1039–1047
Wang J et al (2017) Test-retest reliability of functional connectivity networks during naturalistic fMRI paradigms. Hum Brain Mapp 38:2226–2241
Wang K et al (2007) Altered functional connectivity in early Alzheimer’s disease: a resting-state fMRI study. Hum Brain Mapp 28:967–978
Wang P et al (2021) White matter functional connectivity in resting-state fMRI: robustness, reliability, and relationships to gray matter. Cereb Cortex. https://doi.org/10.1093/cercor/bhab181
Wise SP et al (1997) Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Ann Rev Neurosci 20:25–42
Wu X et al (2009) Multiple neural networks supporting a semantic task: an fMRI study using independent component analysis. Neuroimage 45:1347–1358
**a M, Wang J, He Y (2013) BrainNet viewer: a network visualization tool for human brain connectomics. PLoS ONE 8:e68910
Yeo BTT et al (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165
Yoshida K et al (2019) Serotonin-mediated inhibition of ventral hippocampus is required for sustained goal-directed behavior. Nat Neurosci 22:770–777
Zaborszky L et al (2008) Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage 42:1127–1141
Zanto TP, Pa J, Gazzaley A (2014) Reliability measures of functional magnetic resonance imaging in a longitudinal evaluation of mild cognitive impairment. Neuroimage 84:443–452
Zhu L et al (2014) Temporal reliability and lateralization of the resting-state language network. PLoS One. 9:e85880
Zilles K (2017) Receptors and the default mode network. Lecture presented at the OHBM Annual Meeting. https://www.humanbrainmap**.org/files/2017/ED%20Courses/Course%20Materials/Anatomy_Zilles_Karl.pdf.
Zou L et al (2016) Neural correlates of morphological processing: evidence from Chinese. Front Hum Neurosci 9:714
Acknowledgements
We are indebted to the reviewer and editors for helpful comments on the original version of the study, and to the Research Center for Mind, Brain & Learning at the National Chengchi University for the great assistance during fMRI data acquisition. Much of the data in Table 2 was prepared in 2019 with the assistance of Professor Karl Zilles. This collaboration of collecting cortisol levels was initiated by Professor Zilles, and the main research findings had been derived by the time of his passing. Professor Zilles enthusiastically extolled that “SCIENCE IS NOT FOR SALE”, and promulgated the need to promote neuroscience research around the world, particularly among young scientists. We honor him as one of the pioneers of neuroscience as well as a friend who enriched us with his experience and wisdom. This research was supported by grants MOST-109-2410-H-001-021 and MOST-110-2410-H-001-046 from the Ministry of Science and Technology, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Andrew Zalesky .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chang, SD., Kuo, PC., Zilles, K. et al. Brain Reactions to Opening and Closing the Eyes: Salivary Cortisol and Functional Connectivity. Brain Topogr 35, 375–397 (2022). https://doi.org/10.1007/s10548-022-00897-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-022-00897-x