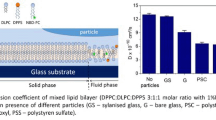
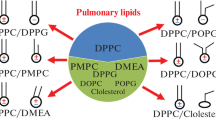
To understand the nature of xenon-induced recovery of the functional activity of pulmonary surfactant during inhalation of a gas mixture of Xe/O2, the mechanisms of the ongoing processes were studied in silico. Impaired ability of pulmonary surfactant to maintain low surface tension preventing alveolar atelectasis occurs due to formation of aggregates of its phospholipids and a decrease in their lateral mobility. Aggregated lipid systems, whose structure can explain the loss of lateral mobility of surfactant phospholipids, were modeled in silico at the molecular level. Changes in the Gibbs energy and enthalpy in the reactions of the formation and decomposition of xenon intermediates with model systems of various compositions/structures were calculated. The simulation was carried out for atomic xenon and for xenon polarized by molecular oxygen in the gas phase and taking into account solvation with water. The loss of lateral mobility of phospholipids can be explained by specific features of electronic structure of hydrophobic hydrocarbon molecules (acyl chains), which, under certain conditions, are capable of forming structured common regions of the electrostatic potential, to which xenon has an affinity. In this case, inclusion coordination compounds of the “guest—host” type are formed, which subsequently decompose due to the nature of the polarization of the Xe atoms. The formation and decomposition of xenon intermediates in these systems lead to recovery of the lateral mobility (fluidity) of phospholipids, which restores functional activity of surfactant films.
Similar content being viewed by others
Change history
13 February 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10517-024-06037-8
References
Hussain M, Khurram Syed S, Fatima M, Shaukat S, Saadullah M, Alqahtani AM, Alqahtani T, Bin Emran T, Alamri AH, Barkat MQ, Wu X. Acute Respiratory Distress Syndrome and COVID-19: A Literature Review. J. Inflamm. Res. 2021;14:7225-7242. doi: https://doi.org/10.2147/JIR.S334043
Wang S, Li Z, Wang X, Zhang S, Gao P, Shi Z. The role of pulmonary surfactants in the treatment of acute respiratory distress syndrome in COVID-19. Front. Pharmacol. 2021;12:698905. doi: https://doi.org/10.3389/fphar.2021.698905
Echaide M, Autilio C, Arroyo R, Perez-Gil J. Restoring pulmonary surfactant membranes and films at the respiratory surface. Biochim. Biophys. Acta Biomembr. 2017;1859(9, Pt B):1725-1739. doi: https://doi.org/10.1016/j.bbamem.2017.03.015
Perez-Gil J, Weaver TE. Pulmonary surfactant pathophysiology: current models and open questions. Physiology (Bethesda). 2010;25(3):132-141. doi: https://doi.org/10.1152/physiol.00006.2010
Rizzo AN, Haeger SM, Oshima K, Yang Y, Wallbank AM, ** Y, Lettau M, McCaig LA, Wickersham NE, McNeil JB, Zakharevich I, McMurtry SA, Langouët-Astrié CJ, Kopf KW, Voelker DR, Hansen KC, Shaver CM, Kerchberger VE, Peterson RA, Kuebler WM, Ochs M, Veldhuizen RA, Smith BJ, Ware LB, Bastarache JA, Schmidt EP. Alveolar epithelial glycocalyx degradation mediates surfactant dysfunction and contributes to acute respiratory distress syndrome. JCI Insight. 2022;7(2):e154573. doi: https://doi.org/10.1172/jci.insight.154573
Knudsen L, Ochs M. The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem. Cell Biol. 2018;150(6):661-676. doi: https://doi.org/10.1007/s00418-018-1747-9
Ochs M, Hegermann J, Lopez-Rodriguez E, Timm S, Nouailles G, Matuszak J, Simmons S, Witzenrath M, Kuebler WM. On top of the alveolar epithelium: surfactant and the glycocalyx. Int. J. Mol. Sci. 2020;21(9):3075. doi: https://doi.org/10.3390/ijms21093075
Udut VV, Naumov SA, Evtushenko DN, Udut EV, Naumov SS, Zyuz’kov GN. A case of xenon inhalation therapy for respiratory failure and neuropsychiatric disorders associated with COVID-19. EXCLI J. 2021;20:1517-1525. doi: https://doi.org/10.17179/excli2021-4316
Udut VV, Naumov SA, Udut EV, Naumov SS, Evtushenko DN, Chumakova ON, Zyuz’kov GN. Mechanisms of the effects of short-term inhalations of Xe and O2 gas mixture in the rehabilitation of post-COVID ventilation failure. Bull. Exp. Biol. Med. 2022;172(3):364-367. doi: https://doi.org/10.1007/s10517-022-05393-7
Rosenberg OA. Lung surfactant and its use in lung diseases. Obshch. Reanimatol. 2007;3(1):66-77. Russian. doi: https://doi.org/10.15360/1813-9779-2007-1-66-77
Ishutsina OV. The surfactant system of the lungs. a review article. Vestn. Vitebsk. Gos. Med. Univ. 2021;20(4):7-17. Russian. doi: https://doi.org/10.22263/2312-4156.2021.4.7
Pérez-Gil J. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid-protein interactions. Biochim. Biophys Acta. 2008;1778(7-8):1676-95. doi: https://doi.org/10.1016/j.bbamem.2008.05.003
Bobrov MF, Tsirelson VG. Chemical bonding in the complexes XEF5+XF6—(X=P, AS, SB, BI). Russ. J. Coordinat. Chem. 2005;31(10):746-756. doi: https://doi.org/10.1007/s11173-005-0158-3
Mazej Z. Noble-gas chemistry more than half a century after the first report of the noble-gas compound. Molecules. 2020;25(13):3014. doi: https://doi.org/10.3390/molecules25133014
Frontera A. Noble gas bonding interactions involving xenon oxides and fluorides. Molecules. 2020;25(15):3419. doi: https://doi.org/10.3390/molecules25153419.
Joseph AI, Lapidus SH, Kane CM, Holman KT. Extreme Confinement of Xenon by Cryptophane-111 in the solid state. Angew Chem. Int. Ed. Engl. 2015;54(5):1471-1475. doi: https://doi.org/10.1002/anie.201409415
Enright GD, Udachin KA, Moudrakovski IL, Ripmeester JA. Thermally programmable gas storage and release in single crystals of an organic van der Waals host. J. Am. Chem. Soc. 2003;125(33):9896-9897. doi: https://doi.org/10.1021/ja0351701
Dunning TH Jr, Hay PJ. Gaussian Basis Sets for Molecular Calculations. Modern Thetoretical Chemistry. Schaefer HF III, ed. New York, 1977. Vol. 3. P. 1-28.
Hay PJ, Wadt WR. Ab initio effective core potentials for molecular calculations — potentials for the transition-metal atoms Sc to Hg. J. Chem. Phys. 1985;82(1):270-283. doi:https://doi.org/10.1063/1.448799
Wadt WR, Hay PJ. Ab initio effective core potentials for molecular calculations — potentials for main group elements Na to Bi. J. Chem. Phys. 1985;82(1):284-298. doi: https://doi.org/10.1063/1.448800
Hay PJ, Wadt WR. Ab initio effective core potentials for molecular calculations — potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985;82(1):299-310. doi: https://doi.org/10.1063/1.448975
Barone V. Characterization of the potential energy surface of the HO2 molecular system by a density functional approach. J. Chem. Phys. 1994;101:10 666-10 676. doi: https://doi.org/10.1063/1.467880
Barone V, Cossi M, Tomasi J. A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. J. Chem. Phys. 1997;107:3210-3221. doi: https://doi.org/10.1063/1.474671
Barone V, Cossi M, Tomasi J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comp. Chem. 1998;19(4):404-417. doi: https://doi.org/10.1002/(SICI)1096-987X(199803)19:4<404::AID-JCC3>3.0.CO;2-W
Dennington R, Keith TA, Millam JM. GaussView 6.0. 16. Semichem Inc., 2016.
Keating E, Zuo YY, Tadayyon SM, Petersen NO, Possmayer F, Veldhuizen RA. A modified squeeze-out mechanism for generating high surface pressures with pulmonary surfactant. Biochim. Biophys. Acta. 2012;1818(5):1225-1234. doi: https://doi.org/10.1016/j.bbamem.2011.12.007
Booker RD, Sum AK. Biophysical changes induced by xenon on phospholipid bilayers. Biochim. Biophys. Acta. 2013;1828(5):1347-1356. doi: https://doi.org/10.1016/j.bbamem.2013.01.016
Enright GD, Udachin KA, Moudrakovski IL, Ripmeester JA. CCDC 221010: Experimental Crystal Structure Determination. Unknown Publisher, 2004. doi: https://doi.org/10.5517/cc7dzcy
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Kletochnye Tekhnologii v Biologii i Meditsine, No. 3, pp. 176-183, September, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Evtushenko, D.N., Fateev, A.V., Naumov, S.A. et al. Xenon-Induced Recovery of Functional Activity of Pulmonary Surfactant (In Silico Study). Bull Exp Biol Med 176, 260–267 (2023). https://doi.org/10.1007/s10517-024-06006-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-024-06006-1