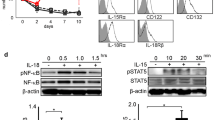
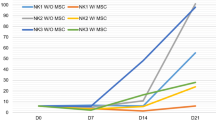
Activated NK cells in appropriate conditions are known to express stem cell antigen 1 (Sca-1/Ly-6A/E). To investigate its production, NK cells isolated from mouse spleens were incubated ex vivo in the presence of different combinations of cytokines (IL-12, IL-15, IL-18, and IFNγ). Expression of Sca-1 was found to be considerably higher in NK cells incubated in the presence of IL-18, IL-15, and IL-12 than in those treated with IL-15 and IL-18 only. To test the hypothesis that the effect of IL-12 was due to stimulation of IFNγ production, we replaced IL-12 with IFNγ in some samples and added specific anti-IFNγ antibody to some samples cultured with IL-15/IL-18+IL-12. In the subpopulations incubated in the presence of IL-15/IL-18 with added IFNγ instead of IL-12, the expression of Sca-1 was not increased. By contrast, in samples treated with IL-15/IL-18+IL-12 and anti-IFNγ antibody, the expression of Sca-1 was activated to a similar extent as in those stimulated by IL-15/IL-18+IL-12 combination without the antibody. The obtained data suggest that IL-12 activates the production of Sca-1 by NK cells through an IFNγ-independent mechanism.
Similar content being viewed by others
References
Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27(45):5932-5943. https://doi.org/10.1038/onc.2008.267
Stanley AC, Lacy P. Pathways for cytokine secretion. Physiology (Bethesda). 2010;25(4):218-229. https://doi.org/10.1152/physiol.00017.2010
Fogel LA, Sun MM, Geurs TL, Carayannopoulos LN, French AR. Markers of nonselective and specific NK cell activation. J. Immunol. 2013;190(12):6269-6276. https://doi.org/10.4049/jimmunol.1202533
Morcos MNF, Schoedel KB, Hoppe A, Behrendt R, Basak O, Clevers HC, Roers A, Gerbaulet A. SCA-1 Expression Level Identifies Quiescent Hematopoietic Stem and Progenitor Cells. Stem Cell Reports. 2017;8(6):1472-1478. https://doi.org/10.1016/j.stemcr.2017.04.012
Whitmire JK, Eam B, Whitton JL. Mice deficient in stem cell antigen-1 (Sca1, Ly-6A/E) develop normal primary and memory CD4+ and CD8+ T-cell responses to virus infection. Eur. J. Immunol. 2009;39(6):1494-1504. https://doi.org/10.1002/eji.200838959
Páral P, Faltusová K, Molík M, Renešová N, Šefc L, Nečas E. Cell cycle and differentiation of Sca-1+ and Sca-1— hematopoietic stem and progenitor cells. Cell. Cycle. 2018;17(16):1979-1991. https://doi.org/10.1080/15384101.2018.1502573
Mitchell PO, Mills T, O’Connor RS, Kline ER, Graubert T, Dzierzak E, Pavlath GK. Sca-1 negatively regulates proliferation and differentiation of muscle cells. Dev. Biol. 2005;283(1):240-252. https://doi.org/10.1016/j.ydbio.2005.04.016
Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25(6):1339-1347. https://doi.org/10.1634/stemcells.2006-0644
Okazawa A, Kanai T, Nakamaru K, Sato T, Inoue N, Ogata H, Iwao Y, Ikeda M, Kawamura T, Makita S, Uraushihara K, Okamoto R, Yamazaki M, Kurimoto M, Ishii H, Watanabe M, Hibi T. Human intestinal epithelial cell-derived interleukin (IL)-18, along with IL-2, IL-7 and IL-15, is a potent synergistic factor for the proliferation of intraepithelial lymphocytes. Clin. Exp. Immunol. 2004;136(2):269-276. https://doi.org/10.1111/j.1365-2249.2004.02431.x
Nandagopal N, Ali AK, Komal AK, Lee SH. The Critical Role of IL-15-PI3K-mTOR Pathway in Natural Killer Cell Effector Functions. Front. Immunol. 2014;5:187. https://doi.org/10.3389/fimmu.2014.00187
French AR, Holroyd EB, Yang L, Kim S, Yokoyama WM. IL-18 acts synergistically with IL-15 in stimulating natural killer cell proliferation. Cytokine. 2006;35(5-6):229-234. https://doi.org/10.1016/j.cyto.2006.08.006
Oka N, Markova T, Tsuzuki K, Li W, El-Darawish Y, Pencheva-Demireva M, Yamanishi K, Yamanishi H, Sakagami M, Tanaka Y, Okamura H. IL-12 regulates the expansion, phenotype, and function of murine NK cells activated by IL-15 and IL-18. Cancer Immunol. Immunother. 2020;69(9):1699-1712. https://doi.org/10.1007/s00262-020-02553-4
He XS, Draghi M, Mahmood K, Holmes TH, Kemble GW, Dekker CL, Arvin AM, Parham P, Greenberg HB. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J. Clin. Invest. 2004;114(12):1812-1819. https://doi.org/10.1172/JCI22797
El-Darawish Y, Li W, Yamanishi K, Pencheva M, Oka N, Yamanishi H, Matsuyama T, Tanaka Y, Minato N, Okamura H. Frontline Science: IL-18 primes murine NK cells for proliferation by promoting protein synthesis, survival, and autophagy. J. Leukoc. Biol. 2018;104(2):253-264. https://doi.org/10.1002/JLB.1HI1017-396RR
de Araújo-Souza PS, Hanschke SC, Viola JP. Epigenetic control of interferon-gamma expression in CD8 T cells. J. Immunol. Res. 2015;2015:849573. https://doi.org/10.1155/2015/849573
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 174, No. 7, pp. 74-78, July, 2022
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Markova, T., Sainova, I., Kolyovska, V. et al. Production of Stem Cell Antigen 1 Sca-1/Ly-6A/E by Freshly Isolated NK Cells Cultured with Relevant Cytokines. Bull Exp Biol Med 174, 62–65 (2022). https://doi.org/10.1007/s10517-022-05649-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-022-05649-2