Abstract
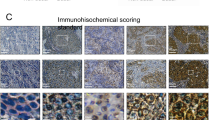
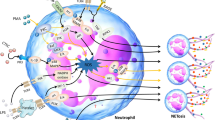
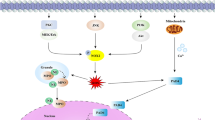
Neutrophil and neutrophil extracellular traps (NETs) were reported to be associated with tumor development, but the exact role and concrete mechanisms are still poorly understood, especially in triple negative breast cancer (TNBC). In this study, our results exhibited that NETs formation in TNBC tissues was higher than that in non-TNBC tissues, and NETs formation was distinctly correlated with tumor size, ki67 level and lymph node metastasis in TNBC patients. Subsequent in vivo experiments demonstrated that NETs inhibition could suppress TNBC tumor growth and lung metastasis. Further in vitro experiments uncovered that oncogenic function of NETs on TNBC cells were possibly dependent on TLR9 expression. We also found that neutrophils from peripheral blood of TNBC patients with postoperative fever were prone to form NETs and could enhance the proliferation and invasion of TNBC cells. Mechanistically, we revealed that NETs could interact with TLR9 to decrease Merlin phosphorylation which contributed to TNBC cell ferroptosis resistance. Our work provides a novel insight into the mechanism of NETs promoting TNBC progression and blocking the key modulator of NETs might be a promising therapeutic strategy in TNBC.






Similar content being viewed by others
Data availability
The data are available within the Article, Supplementary Information, or available from the authors upon request. Source data are provided with this paper.
References
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363(20):1938–1948
Wang C et al (2018) Triple negative breast cancer in Asia: an insider’s view. Cancer Treat Rev 62:29–38
Albrengues J et al (2018) Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361(6409):eaa04227
Asano Y et al (2016) Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple-negative breast cancer. Ann Surg Oncol 23(4):1104–1110
Brinkmann V et al (2004) Neutrophil extracellular traps kill bacteria. Science 303(5663):1532–1535
Park J et al (2016) Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med 8(361):361ra38
Ren K et al (2018) Prognostic role of derived neutrophil-to-lymphocyte ratio in surgical triple-negative breast cancer. Cancer Manag Res 10:4891–4898
Teucher G, Schindler AE (1987) Postoperative fever and prognosis in breast cancer. Arch Geschwulstforsch 57(4):309–317
Warnatsch A et al (2015) Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349(6245):316–320
Tohme S et al (2016) Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res 76(6):1367–1380
**a X et al (2022) Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat Commun 13(1):1017
Yazdani HO et al (2019) Neutrophil extracellular traps drive mitochondrial homeostasis in tumors to augment growth. Cancer Res 79(21):5626–5639
Yang XM et al (2018) Overexpression of Rac GTPase activating protein 1 contributes to proliferation of cancer cells by reducing hippo signaling to promote cytokinesis. Gastroenterology 155(4):1233-1249.e22
Cooper J, Giancotti FG (2017) Cancer: a new role for non-canonical Hippo signaling. Cell Res 27(4):459–460
Heidary Arash E et al (2017) MARK4 inhibits Hippo signaling to promote proliferation and migration of breast cancer cells. EMBO Rep 18(3):420–436
Wang C et al (2015) The interplay between TEAD4 and KLF5 promotes breast cancer partially through inhibiting the transcription of p27Kip1. Oncotarget 6(19):17685–17697
Martins-Cardoso K et al (2020) Neutrophil extracellular traps (NETs) promote pro-metastatic phenotype in human breast cancer cells through epithelial-mesenchymal transition. Cancers (Basel) 12(6):1542
Papayannopoulos V (2018) Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18(2):134–147
Zahid A et al (2020) Molecular and structural basis of DNA sensors in antiviral innate immunity. Front Immunol 11:613039
Mota M, Shevde LA (2020) Merlin regulates signaling events at the nexus of development and cancer. Cell Commun Signal 18(1):63
Qi JL et al (2020) Pulmonary Staphylococcus aureus infection regulates breast cancer cell metastasis via neutrophil extracellular traps (NETs) formation. MedComm 1(2):188–201
Castanheira FVS, Kubes P (2019) Neutrophils and NETs in modulating acute and chronic inflammation. Blood 133(20):2178–2185
Hahn J et al (2016) Neutrophils and neutrophil extracellular traps orchestrate initiation and resolution of inflammation. Clin Exp Rheumatol 34(4 Suppl 98):6–8
Zhu B et al (2021) NF-kappaB and neutrophil extracellular traps cooperate to promote breast cancer progression and metastasis. Exp Cell Res 405(2):112707
Yang L et al (2020) DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 583(7814):133–138
Demers M et al (2012) Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA 109(32):13076–13081
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Nie M et al (2019) Neutrophil extracellular traps induced by IL8 promote diffuse large B-cell lymphoma progression via the TLR9 signaling. Clin Cancer Res 25(6):1867–1879
Ding Y et al (2021) Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. J Hematol Oncol 14(1):19
Song X et al (2020) Role of GPX4-mediated ferroptosis in the sensitivity of triple negative breast cancer cells to gefitinib. Front Oncol 10:597434
Yu M et al (2019) Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci 110(10):3173–3182
Zhang Z et al (2021) Holo-lactoferrin: the link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics 11(7):3167–3182
Mota M et al (2021) Merlin deficiency alters the redox management program in breast cancer. Mol Oncol 15(4):942–956
Couderc C et al (2016) AMOTL1 promotes breast cancer progression and is antagonized by merlin. Neoplasia 18(1):10–24
Acknowledgements
The patients are gratefully acknowledged for their participation in this study.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82002777), Multidisciplinary Cross Research Foundation of Shanghai Jiao Tong University (No. YG2019QNA26), Shanghai Sailing Program (22YF1424500).
Author information
Authors and Affiliations
Contributions
HD, YD, YS conceived of the study and participated in its design and coordination. LY, XD, XS, YL and WZ performed molecular biological experiments. HD, XS, XD, YD collected samples. LY, HD, YS contributed to data acquisition and analysis. XS, XM, YS performed the statistical analyses for all the data. HD, XS drafted the manuscript. LY, YD, YS, HD, XM revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests with the contents of this article.
Ethical approval
The studies involving human participants were reviewed and approved by The Affiliated Huaian Hospital of Xuzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, L., Sheng, X., Dong, X. et al. Neutrophil extracellular traps mediate TLR9/Merlin axis to resist ferroptosis and promote triple negative breast cancer progression. Apoptosis 28, 1484–1495 (2023). https://doi.org/10.1007/s10495-023-01866-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01866-w