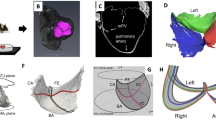
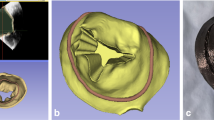
Abstract
Aortic valve (AV) disease is a common valvular lesion in the United States, present in about 5% of the population at age 65 with increasing prevalence with advancing age. While current replacement heart valves have extended life for many, their long-term use remains hampered by limited durability. Non-surgical treatments for AV disease do not yet exist, in large part because our understanding of AV disease etiology remains incomplete. The direct study of human AV disease remains hampered by the fact that clinical data is only available at the time of treatment, where the disease is at or near end stage and any time progression information has been lost. Large animal models, long used to assess replacement AV devices, cannot yet reproduce AV disease processes. As an important alternative mouse animal models are attractive for their ability to perform genetic studies of the AV disease processes and test potential pharmaceutical treatments. While mouse models have been used for cellular and genetic studies of AV disease, their small size and fast heart rates have hindered their use for tissue- and organ-level studies. We have recently developed a novel ex vivo micro-CT-based methodology to 3D reconstruct murine heart valves and estimate the leaflet mechanical behaviors (Feng et al. in Sci Rep 13(1):12852, 2023). In the present study, we extended our approach to 3D reconstruction of the in vivo functional murine AV (mAV) geometry using high-frequency four-dimensional ultrasound (4DUS). From the resulting 4DUS images we digitized the mAV mid-surface coordinates in the fully closed and fully opened states. We then utilized matched high-resolution µCT images of ex vivo mouse mAV to develop mAV NURBS-based geometric model. We then fitted the mAV geometric model to the in vivo data to reconstruct the 3D in vivo mAV geometry in the closed and open states in n = 3 mAV. Results demonstrated high fidelity geometric results. To our knowledge, this is the first time such reconstruction was ever achieved. This robust assessment of in vivo mAV leaflet kinematics in 3D opens up the possibility for longitudinal characterization of murine models that develop aortic valve disease.













Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Lerman, D. A., S. Prasad, and N. Alotti. Calcific aortic valve disease: molecular mechanisms and therapeutic approaches. Eur. Cardiol. Rev. 10(2):108, 2015.
Tasoudis, P. T., D. N. Varvoglis, E. Vitkos, K. S. Mylonas, M. P. Sa, J. S. Ikonomidis, T. G. Caranasos, and T. Athanasiou. Mechanical versus bioprosthetic valve for aortic valve replacement: systematic review and meta-analysis of reconstructed individual participant data. Eur. J. Cardio-Thorac. Surg. 62(1):ezac268, 2022.
Kheradvar, A., R. Zareian, S. Kawauchi, R. L. Goodwin, and S. Rugonyi. Animal models for heart valve research and development. Drug Discov. Today. 24:55–62, 2017.
Cheek, J. D., E. E. Wirrig, C. M. Alfieri, J. F. James, and K. E. Yutzey. Differential activation of valvulogenic, chondrogenic, and osteogenic pathways in mouse models of myxomatous and calcific aortic valve disease. J. Mol. Cell. Cardiol. 52(3):689–700, 2012.
Lincoln, J., C. M. Alfieri, and K. E. Yutzey. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev. Dyn. 230(2):239–250, 2004. https://doi.org/10.1002/dvdy.20051.
Colvee, E., and J. M. Hurle. Maturation of the extracellular material of the semilunar heart valves in the mouse. Anatomy Embryol. 162:343–352, 1981.
Rego, B. V., A. M. Pouch, J. H. Gorman, R. C. Gorman, and M. S. Sacks. Patient-specific quantification of normal and bicuspid aortic valve leaflet deformations from clinically derived images. Ann. Biomed. Eng. 50:1–15, 2022. https://doi.org/10.1007/s10439-021-02882-0.
Rotman, O. M., M. Bianchi, R. P. Ghosh, B. Kovarovic, and D. Bluestein. Principles of tavr valve design, modelling, and testing. Expert Rev Med. Devices. 15:771–791, 2018. https://doi.org/10.1080/17434440.2018.1536427.
Emendi, M., F. Sturla, R. P. Ghosh, M. Bianchi, F. Piatti, F. R. Pluchinotta, D. Giese, M. Lombardi, A. Redaelli, and D. Bluestein. Patient-specific bicuspid aortic valve biomechanics: a magnetic resonance imaging integrated fluid–structure interaction approach. Ann. Biomed. Eng. 49:627–641, 2021.
Lavon, K., A. Morany, R. Halevi, A. Hamdan, E. Raanani, D. Bluestein, and R. Haj-Ali. Progressive calcification in bicuspid valves: a coupled hemodynamics and multiscale structural computations. Ann. Biomed. Eng. 49:3310–3322, 2021.
Pouch, A. M., B. M. Jackson, P. A. Yushkevich, J. H. Gorman 3rd., and R. C. Gorman. 4d-transesophageal echocardiography and emerging imaging modalities for guiding mitral valve repair. Ann. Cardiothorac. Surg. 4(5):461–462, 2015. https://doi.org/10.3978/j.issn.2225-319X.2015.02.01.
Pouch, A. M., C. Xu, P. A. Yushkevich, A. S. Jassar, M. Vergnat, J. H. Gorman 3rd., R. C. Gorman, C. M. Sehgal, and B. M. Jackson. Semi-automated mitral valve morphometry and computational stress analysis using 3d ultrasound. J. Biomech. 45(5):903–907, 2012. https://doi.org/10.1016/j.jbiomech.2011.11.033.
Sewell-Loftin, M.-K., C. B. Brown, H. S. Baldwin, and W. D. Merryman. Novel technique for quantifying mouse heart valve leaflet stiffness with atomic force microscopy. J. Heart Valve Dis. 21(4):513, 2012.
Sacks, M. S., and A. P. Yoganathan. Heart valve function: a biomechanical perspective. Philos. Trans. R. Soc. B. 362(1484):1369–1391, 2007.
Sacks, M. S., W. D. Merryman, and D. E. Schmidt. On the biomechanics of heart valve function. J. Biomech. 42:1804–1824, 2009.
Feng, X., Y. Liu, D. Kamensky, D. W. McComb, C. K. Breuer, and M. S. Sacks. Functional mechanical behavior of the murine pulmonary heart valve. Sci. Rep. 13(1):12852, 2023.
Liu, Y., X. Feng, H. Liu, D. McComb, C. Breuer, and M. Sacks. On the shape and structure of the murine pulmonary heart valve. Sci. Rep. 11(1):14078, 2021.
Damen, F. W., A. G. Berman, A. H. Soepriatna, J. M. Ellis, S. D. Buttars, K. L. Aasa, and C. J. Goergen. High-frequency 4-dimensional ultrasound (4dus): a reliable method for assessing murine cardiac function. Tomography. 3(4):180–187, 2017.
Aggarwal, A. An improved parameter estimation and comparison for soft tissue constitutive models containing an exponential function. Biomech. Model. Mechanobiol. 16(4):1309–1327, 2017. https://doi.org/10.1007/s10237-017-0889-3.
Aggarwal, A., A. M. Pouch, E. Lai, J. Lesicko, P. A. Yushkevich, J. H. Gorman III., R. C. Gorman, and M. S. Sacks. In-vivo heterogeneous functional and residual strains in human aortic valve leaflets. J. Biomech. 2016. https://doi.org/10.1016/j.jbiomech.2016.04.038.
Aggarwal, A., and M. S. Sacks. An inverse modeling approach for semilunar heart valve leaflet mechanics: exploitation of tissue structure. Biomech. Model. Mechanobiol. 1–24, 2005.
Damen, F., A. Adelsperger, K. Wilson, and C. Goergen. Comparison of traditional and integrated digital anesthetic vaporizers. J. Am. Assoc. Lab. Anim. 54(756–762):390, 2015.
Damen, F. W., J. P. Salvas, A. S. Pereyra, J. M. Ellis, and C. J. Goergen. Muscle mechanics and ventricular function: Improving characterization of hypertrophy-induced murine cardiac dysfunction using four-dimensional ultrasound-derived strain map**. Am. J. Physiol.-Heart Circ. Physiol. 321(1):H197, 2021.
Piegl, L., and W. Tiller. The NURBS Book. New York: Springer, 2012.
Aggarwal, A., A. M. Pouch, E. Lai, J. Lesicko, P. A. Yushkevich, J. H. GormanIii, R. C. Gorman, and M. S. Sacks. In-vivo heterogeneous functional and residual strains in human aortic valve leaflets. J. Biomech. 49:2481–2490, 2016. https://doi.org/10.1016/j.jbiomech.2016.04.038.
Aggarwal, A., and M. S. Sacks. An inverse modeling approach for semilunar heart valve leaflet mechanics: exploitation of tissue structure. Biomech. Model. Mechanobiol. 15:909–932, 2016. https://doi.org/10.1007/s10237-015-0732-7.
Otto, C. M. Calcific aortic valve disease: new concepts. Semin. Thorac. Cardiovasc. Surg. 22(4):276–284, 2010. https://doi.org/10.1053/j.semtcvs.2011.01.009.
Freeman, R. V., and C. M. Otto. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 111(24):3316–3326, 2005. https://doi.org/10.1161/circulationaha.104.486738.
Freeman, R. V., and C. M. Otto. Management of asymptomatic valvular aortic stenosis. Indian Heart J. 54(1):31–38, 2002.
Kurtz, C. E., and C. M. Otto. Aortic stenosis: clinical aspects of diagnosis and management, with 10 illustrative case reports from a 25-year experience. Medicine. 89(6):349–379, 2010. https://doi.org/10.1097/MD.0b013e3181fe5648.
Beckmann, E., J. B. Grau, R. Sainger, P. Poggio, and G. Ferrari. Insights into the use of biomarkers in calcific aortic valve disease. J. Heart Valve Dis. 19(4):441–452, 2010.
Grau, J. B., P. Poggio, R. Sainger, W. J. Vernick, W. F. Seefried, E. Branchetti, B. C. Field, J. E. Bavaria, M. A. Acker, and G. Ferrari. Analysis of osteopontin levels for the identification of asymptomatic patients with calcific aortic valve disease. Ann. Thorac. Surg. 93(1):79–86, 2012. https://doi.org/10.1016/j.athoracsur.2011.08.036.
Yu, P.-J., A. Skolnick, G. Ferrari, K. Heretis, P. Mignatti, G. Pintucci, B. Rosenzweig, J. Diaz-Cartelle, I. Kronzon, G. Perk, H. I. Pass, A. C. Galloway, E. A. Grossi, and J. B. Grau. Correlation between plasma osteopontin levels and aortic valve calcification: potential insights into the pathogenesis of aortic valve calcification and stenosis. J. Thorac. Cardio-Vasc. Surg. 138(1):196–199, 2009. https://doi.org/10.1016/j.jtcvs.2008.10.045.
Parolari, A., E. Tremoli, L. Cavallotti, M. Trezzi, S. Kassem, C. Loardi, F. Veglia, G. Ferrari, D. Pacini, and F. Alamanni. Do statins improve outcomes and delay the progression of non-rheumatic calcific aortic stenosis? Heart. 97(7):523–529, 2011. https://doi.org/10.1136/hrt.2010.215046.
Cowell, S. J., D. E. Newby, R. J. Prescott, P. Bloomfield, J. Reid, D. B. Northridge, and N. A. Boon. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N. Engl. J. Med. 352(23):2389–2397, 2005. https://doi.org/10.1056/NEJMoa043876.
Benton, J. A., H. B. Kern, L. A. Leinwand, P. D. Mariner, and K. S. Anseth. Statins block calcific nodule formation of valvular interstitial cells by inhibiting alpha-smooth muscle actin expression. Arterioscler. Thromb. Vasc. Biol. 29(11):1950–1957, 2009. https://doi.org/10.1161/atvbaha.109.195271.
Rossebo, A. B., T. R. Pedersen, K. Boman, P. Brudi, J. B. Chambers, K. Egstrup, E. Gerdts, C. Gohlke-Barwolf, I. Holme, Y. A. Kesaniemi, W. Malbecq, C. A. Nienaber, S. Ray, T. Skjaerpe, K. Wachtell, and R. Willenheimer. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med. 359(13):1343–1356, 2008. https://doi.org/10.1056/NEJMoa0804602.
Rajamannan, N. M. Calcific aortic stenosis: medical and surgical management in the elderly. Curr. Treat. Options Cardiovasc. Med. 7(6):437–442, 2005. https://doi.org/10.1007/s11936-005-0028-9.
Mulholland, D. L., and A. I. Gotlieb. Cell biology of valvular interstitial cells. Can. J. Cardiol. 12(3):231–236, 1996.
Schoen, F. J. Cardiac valves and valvular pathology: update on function, disease, repair, and replacement. Cardiovasc. Pathol. 14(4):189–194, 2005.
Vesely, I., J. E. Barber, and N. B. Ratliff. Tissue damage and calcification may be independent mechanisms of bioprosthetic heart valve failure. J. Heart Valve Dis. 10(4):471–477, 2001.
Sacks, M. S., and F. J. Schoen. Collagen fiber disruption occurs independent of calcification in clinically explanted bioprosthetic heart valves. J. Biomed. Mater. Res. 62(3):359–371, 2002.
Bonow, R. O., B. A. Carabello, K. Chatterjee, A. C. de Leon, Jr., D. P. Faxon, M. D. Freed, W. H. Gaasch, B. W. Lytle, R. A. Nishimura, P. T. O’Gara, R. A. O’Rourke, C. M. Otto, P. M. Shah, J. S. Shanewise, S. C. Smith, Jr., A. K. Jacobs, C. D. Adams, J. L. Anderson, E. M. Antman, V. Fuster, J. L. Halperin, L. F. Hiratzka, S. A. Hunt, R. Nishimura, R. L. Page, B. Riegel, ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 48(3):e1–148, 2006. https://doi.org/10.1016/j.jacc.2006.05.021
Guidoin, R., Y. Douville, M. A. Clavel, Z. Zhang, M. Nutley, P. Pibarot, and G. Dionne. The marvel of percutaneous cardiovascular devices in the elderly. Ann. N. Y. Acad. Sci. 1197:188–199, 2010. https://doi.org/10.1111/j.1749-6632.2010.05517.x.
Sider, K. L., M. C. Blaser, C. A. Simmons, et al. Animal models of calcific aortic valve disease. Int. J. Inflamm.2011(1):364310, 2011.
Kaplan, S. R., G. Bashein, F. H. Sheehan, M. E. Legget, B. Munt, X. N. Li, M. Sivarajan, E. L. Bolson, M. Zeppa, M. Z. Arch, and R. W. Martin. Three-dimensional echocardiographic assessment of annular shape changes in the normal and regurgitant mitral valve. Am. Heart J. 139(3):378–387, 2000.
Chakraborty, S., J. Cheek, B. Sakthivel, B. J. Aronow, and K. E. Yutzey. Shared gene expression profiles in develo** heart valves and osteoblast progenitor cells. Physiol. Genomics. 35(1):75–85, 2008. https://doi.org/10.1152/physiolgenomics.90212.2008.
Hinton, R. B., Jr., J. Lincoln, G. H. Deutsch, H. Osinska, P. B. Manning, D. W. Benson, and K. E. Yutzey. Extracellular matrix remodeling and organization in develo** and diseased aortic valves. Circ. Res. 98(11):1431–1438, 2006. https://doi.org/10.1161/01.RES.0000224114.
Acknowledgments
This work was supported by the National Institutes of Health Grant HL142504.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Craig Goergen is a paid consultant of FUJIFILM VisualSonics Inc. The other authors declare no conflict of interest.
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gramling, D.P., van Veldhuisen, A.L., Damen, F.W. et al. In Vivo Three-Dimensional Geometric Reconstruction of the Mouse Aortic Heart Valve. Ann Biomed Eng (2024). https://doi.org/10.1007/s10439-024-03555-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10439-024-03555-4