Abstract
Background
This randomized study was designed to evaluate the clinical effect of an elemental diet during chemotherapy in patients with esophageal cancer.
Methods
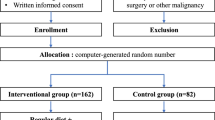
The inclusion criteria were as follows: (1) esophageal squamous cell carcinoma, (2) stage IB-IV, (3) schedule to receive docetaxel, cisplatin, and 5-fluorouracil (DCF chemotherapy), (4) 20–80 years old, (5) performance status of 0–2, (6) oral intake ability, and (7) written informed consent. Patients were divided into two groups: the elemental supplementary group and the non-supplementary group. Patients received ELENTAL® (160 g/day) orally 9 weeks after the start of chemotherapy. Primary endpoint was the incidence of grade 2 or higher gastrointestinal toxicity according to the Common Terminology Criteria for Adverse Events, version 4.0. Secondary endpoints were the incidence of all adverse events and the evaluation of nutritional status.
Results
Thirty-six patients in the elemental supplementary group and 35 patients in the non-supplementary group were included in the analysis. The incidence of grade 2 or higher gastrointestinal toxicity and all grade 3 or 4 adverse events did not differ significantly between the groups. In the elemental supplementary group, the body weight (p = 0.057), muscle mass (p = 0.056), and blood levels of transferrin (p = 0.009), total amino acids (p = 0.019), and essential amino acids (p = 0.006) tended to be maintained after chemotherapy.
Conclusion
Nutritional support provided by an amino acid-rich elemental diet was ineffective for reducing the incidence of adverse events caused by DCF chemotherapy in patients with esophageal cancer.

Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Kato K, Nakajima TE, Ito Y, et al. Phase II study of concurrent chemoradiotherapy at the dose of 50.4 Gy with elective nodal irradiation for stage II–III esophageal carcinoma. Jpn J Clin Oncol. 2013;43:608–15.
Zenda S, Kojima T, Kato K, et al. Multicenter phase 2 study of cisplatin and 5-fluorouracil with concurrent radiation therapy as an organ preservation approach in patients with squamous cell carcinoma of the cervical esophagus. Int J Radiat Oncol Biol Phys. 2016;96:976–84.
Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68–74.
Hara H, Tahara M, Daiko H, et al. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1455–60.
Yamashita K, Hosoda K, Moriya H, et al. Prognostic advantage of docetaxel/cisplatin/ 5-fluorouracil neoadjuvant chemotherapy in clinical stage II/III esophageal squamous cell carcinoma due to excellent control of preoperative disease and postoperative lymph node recurrence. Oncology. 2017;92:221–8.
Satake H, Tahara M, Mochizuki S, et al. A prospective, multicenter phase I/II study of induction chemotherapy with docetaxel, cisplatin and fluorouracil (DCF) followed by chemoradiotherapy in patients with unresectable locally advanced esophageal carcinoma. Cancer Chemother Pharmacol. 2016;78:91–9.
Yokota T, Kato K, Hamamoto Y, et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer. 2016;115:1328–34.
Hironaka S, Tsubosa Y, Mizusawa J, et al. Phase I/II trial of 2-weekly docetaxel combined with cisplatin plus fluorouracil in metastatic esophageal cancer (JCOG0807). Cancer Sci. 2014;105:1189–95.
Sugawara M, Katada C, Katada N, et al. Retrospective evaluation of adverse events of neoadjuvant or induction chemotherapy with docetaxel, cisplatin, and 5-fluorouracil in esophageal squamous cell carcinoma. Esophagus. 2013;10:65–9.
Tanaka Y, Ueno T, Yoshida N, et al. The effect of an elemental diet on oral mucositis of esophageal cancer patients treated with DCF chemotherapy: a multi-center prospective feasibility study (EPOC study). Esophagus. 2018;15:239–48.
Kawashima R, Kawakami F, Maekawa T, et al. Elemental diet moderates 5-fluorouracil-induced gastrointestinal mucositis through mucus barrier alteration. Cancer Chemother Pharmacol. 2015;76:269–77.
Kawashima R, Fujimaki M, Ikenoue Y, et al. Influence of an elemental diet on 5-fluorouracil-induced morphological changes in the mouse salivary gland and colon. Support Care Cancer. 2016;24:1609–16.
Kadota T, Abe S, Yoda Y, et al. Clinical outcomes according to the modified endoscopic criteria for neoadjuvant chemotherapy in resectable esophageal squamous cell carcinoma. Dig Endosc. 2020;32:337–45.
Andou A, Hisamatsu T, Okamoto S, et al. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophages. Gastroenterology. 2009;136:564–74.
Rhoads JM, Chen W, Gookin J, et al. Arginine stimulates intestinal cell migration through a focal adhesion kinase dependent mechanism. Gut. 2004;53:514–22.
Ishikawa T, Yasuda T, Doi T, et al. The amino acid-rich elemental diet Elental® preserves lean body mass during chemo- or chemoradiotherapy for esophageal cancer. Oncol Rep. 2016;36:1093–100.
Okada T, Nakajima Y, Nishikage T, et al. A prospective study of nutritional supplementation for preventing oral mucositis in cancer patients receiving chemotherapy. Asia Pac J Clin Nutr. 2017;26:42–8.
Tanaka Y, Takahashi T, Yamaguchi K, et al. Elemental diet plus glutamine for the prevention of mucositis in esophageal cancer patients receiving chemotherapy: a feasibility study. Support Care Cancer. 2016;24:933–41.
Harada K, Ferdous T, Horinaga D, et al. Efficacy of elemental diet on prevention for chemoradiotherapy-induced oral mucositis in patients with oral squamous cell carcinoma. Support Care Cancer. 2016;24:953–9.
Toyomasu Y, Mochiki E, Yanai M, et al. A prospective pilot study of an elemental nutritional supplement for prevention of oral mucositis during S-1 adjuvant chemotherapy for gastric cancer. Surg Oncol. 2019;29:97–101.
Ogata Y, Ishibashi N, Yamaguchi K, et al. Preventive effects of amino-acid-rich elemental diet Elental® on chemotherapy-induced oral mucositis in patients with colorectal cancer: a prospective pilot study. Support Care Cancer. 2016;24:783–9.
Leitao RF, Ribeiro RA, Lira AM, et al. Glutamine and alanyl-glutamine accelerate the recovery from 5-fluorouracil-induced experimental oral mucositis in hamster. Cancer Chemother Pharmacol. 2008;61:215–22.
Anderson PM, Schroeder G, Skubitz KM. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer. 1998;83:1433–9.
Huang EY, Leung SW, Wang CJ, et al. Oral glutamine to alleviate radiation-induced oral mucositis: a pilot randomized trial. Int J Radiat Oncol Biol Phys. 2000;46:535–9.
Lundholm K, Gunnebo L, Körner U, et al. Effects by daily long term provision of ghrelin to unselected weight-losing cancer patients: a randomized double-blind study. Cancer. 2010;116:2044–52.
Yamamoto K, Takiguchi S, Miyata H, et al. Randomized phase II study of clinical effects of ghrelin after esophagectomy with gastric tube reconstruction. Surgery. 2010;148:31–8.
Ishikawa T, Kitaura Y, Kadota Y, et al. Muscle-specific deletion of BDK amplifies loss of myofibrillar protein during protein undernutrition. Sci Rep. 2017;7:39825.
Cox S, Powell C, Carter B, et al. Role of nutritional status and intervention in oesophageal cancer treated with definitive chemoradiotherapy: outcomes from SCOPE1. Br J Cancer. 2016;115:172–7.
Di Fiore A, Lecleire S, Gangloff A, et al. Impact of nutritional parameter variations during definitive chemoradiotherapy in locally advanced oesophageal cancer. Dig Liver Dis. 2014;46:270–5.
Reisinger KW, Bosmans JW, Uittenbogaart M, et al. Loss of skeletal muscle mass during neoadjuvant chemoradiotherapy predicts postoperative mortality in esophageal cancer surgery. Ann Surg Oncol. 2015;22:4445–52.
Author information
Authors and Affiliations
Contributions
Conception and design: CK, SF, MS, YS, KT, AT, TS, TI, MS, ST, WK. Collection and assembly of data: CK, SF, MS, YS, KT, AT, AW, TW, KI, YF, HH, KH, KY, NH. Data analysis and interpretation: CK, SF, MS, YS, KT, AT, HH, KH, KY, NH. Manuscript writing: CK, SF, MS, YS. Final approval of manuscript: all authors. Financial support: none. Administrative support: CK, SF, MS, YS, KT, AT. Provision of study materials or patients: all authors.
Corresponding author
Ethics declarations
Ethical statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute was obtained from all patients included in the study.
Conflict of interest
There is no financial support for the present study. The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Katada, C., Fukazawa, S., Sugawara, M. et al. Randomized study of prevention of gastrointestinal toxicities by nutritional support using an amino acid-rich elemental diet during chemotherapy in patients with esophageal cancer (KDOG 1101). Esophagus 18, 296–305 (2021). https://doi.org/10.1007/s10388-020-00787-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-020-00787-w