Abstract
Purpose
Having previously demonstrated the efficacy of 0.01% atropine eye drops for inhibiting progression of childhood myopia, we conducted additional analyses to assess post-treatment changes in myopia progression.
Study design
Analysis of follow-up data from a previously reported randomized controlled trial
Methods
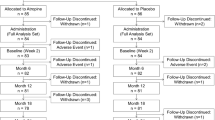
A mixed-effects model was used to compare intergroup changes in spherical equivalent (SE) and axial length (AL) at 1 month and 12 months after discontinuation of 2-year treatment with atropine or placebo in 167 school-age children.
Results
Follow-up measurements were available for 149 participants at 1 month after discontinuation of treatment and for 51 participants at 12 months after discontinuation. At 1 month post-treatment, differences between the atropine and placebo groups in least squares (LS) mean changes in SE and AL, respectively, from 24 months were −0.06 diopters (D) (95% CI: −0.21, 0.08; P = .39) and 0.02 mm (95% CI: −0.05, 0.08; P = .60). At 12 months post-treatment, intergroup differences (atropine vs placebo) in LS mean changes in SE and AL, respectively, were −0.13 D (95% CI: −0.35, 0.10; P = .26) and −0.02 mm (95% CI: −0.12, 0.09; P = .75). LS mean changes in SE and AL from treatment discontinuation did not differ between the groups at 1 or 12 months post-treatment.
Conclusion
Axial elongation was significantly less in the atropine group than in the placebo group. The suppression effect obtained at 2 years was maintained after 12 months. The absence of intergroup differences in myopia progression since treatment cessation suggests that myopic rebound did not occur.



Similar content being viewed by others
References
Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42.
**e Z, Long Y, Wang J, Li Q, Zhang Q. Prevalence of myopia and associated risk factors among primary students in Chongqing: multilevel modeling. BMC Ophthalmol. 2020;20:146.
Bullimore MA, Brennan NA. Myopia control: why each diopter matters. Optom Vis Sci. 2019;96:463–5.
Bullimore MA, Ritchey ER, Shah S, Leveziel N, Bourne RRA, Flitcroft DI. The risks and benefits of myopia control. Ophthalmology. 2021;128:1561–79.
Wang WY, Chen C, Chang J, Chien L, Shih YF, Lin LLK, et al. Pharmacotherapeutic candidates for myopia: a review. Biomed Pharmacother. 2021;133: 111092.
Walline JJ, Lindsley K, Vedula SS, Cotter SA, Mutti DO, Twelker JD. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2011. https://doi.org/10.1002/14651858.CD004916.pub3:CD004916.
Fang YT, Chou YJ, Pu C, Lin PJ, Liu TL, Huang N, et al. Prescription of atropine eye drops among children diagnosed with myopia in Taiwan from 2000 to 2007: a nationwide study. Eye (Lond). 2013;27:418–24.
Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119:347–54.
Yam JC, Jiang Y, Tang SM, Law AKP, Chan JJ, Wong E, et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126:113–24.
Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113:2285–91.
Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116:572–9.
Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stop** atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014;157:451-7.e1.
Brennan NA, Toubouti YM, Cheng X, Bullimore MA. Efficacy in myopia control. Prog Retin Eye Res. 2021;83: 100923.
Chia A, Lu QS, Tan D. Five-year clinical trial on Atropine for the Treatment of Myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016;123:391–9.
Hieda O, Hiraoka T, Fujikado T, Ishiko S, Hasebe S, Torii H, et al. Efficacy and safety of 0.01% atropine for prevention of childhood myopia in a 2-year randomized placebo-controlled study. Jpn J Ophthalmol. 2021;65:315–25.
Salazar M, Shimada K, Patil PN. Iris pigmentation and atropine mydriasis. J Pharmacol Exp Ther. 1976;197:79–88.
Wei S, Li SM, An W, Du J, Liang X, Sun Y, et al. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol. 2020;138:1178–84.
Yam JC, Zhang XJ, Zhang Y, Wang YM, Tang SM, Li FF, et al. Three-Year Clinical Trial of Low-Concentration Atropine for Myopia Progression (LAMP) Study: continued versus washout: phase 3 report. Ophthalmology. 2022;129:308–21.
Hiraoka T, Kakita T, Okamoto F, Takahashi H, Oshika T. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Invest Ophthalmol Vis Sci. 2012;53:3913–9.
Kakita T, Hiraoka T, Oshika T. Influence of overnight orthokeratology on axial elongation in childhood myopia. Investig Ophthalmol Vis Sci. 2011;52:2170–4.
Chamberlain P, Bradley A, Arumugam B, Hammond D, McNally J, Logan NS, et al. Long-term effect of dual-focus contact lenses on myopia progression in children: a 6-year multicenter clinical trial. Optom Vis Sci. 2022;99:204–12.
Lam CSY, Tang WC, Tse DY, Lee RPK, Chun RKM, Hasegawa K, et al. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol. 2020;104:363–8.
Jiang Y, Zhu Z, Tan X, Kong X, Zhong H, Zhang J, et al. Effect of repeated low-level red-light therapy for myopia control in children: a multicenter randomized trial. Ophthalmology. 2022;129:509–19.
Kinoshita N, Konno Y, Hamada N, Kanda Y, Shimmura-Tomita M, Kaburaki T, et al. Efficacy of combined orthokeratology and 0.01% atropine solution for slowing axial elongation in children with myopia: a 2-year randomised trial. Sci Rep. 2020;10:12750.
Xu S, Li Z, Zhao W, Zheng B, Jiang J, Ye G, et al. Effect of atropine, orthokeratology and combined treatments for myopia control: a 2-year stratified randomised clinical trial. Br J Ophthalmol. 2022. https://doi.org/10.1136/bjo-2022-321272.
Sander BP, Collins MJ, Read SA. The effect of topical adrenergic and anticholinergic agents on the choroidal thickness of young healthy adults. Exp Eye Res. 2014;128:181–9.
Yam JC, Jiang Y, Lee J, Li S, Zhang Y, Sun W, et al. The association of choroidal thickening by atropine with treatment effects for myopia: two-year clinical trial of the LAMP Study. Am J Ophthalmol. 2022;237:130–8.
Fu A, Stapleton F, Wei L, Wang W, Zhao B, Watt K, et al. Effect of low-dose atropine on myopia progression, pupil diameter and accommodative amplitude: low-dose atropine and myopia progression. Br J Ophthalmol. 2020;104:1535–41.
Ye L, Shi Y, Yin Y, Li S, He J, Zhu J, et al. Effects of atropine treatment on choroidal thickness in myopic children. Investig Ophthalmol Vis Sci. 2020;61:15–25.
Acknowledgements
The ATOM-J Study was supported by Eye-Lens Pte., Ltd., Singapore. The sponsor had no role in the design or conduct of this research. The authors wish to recognize the contributions of all members of the ATOM-J Study group (Osamu Hieda, Takahiro Hiraoka, Takashi Fujikado, Satoshi Ishiko, Satoshi Hasebe, Hidemasa Torii, Hiroshi Takahashi, Yo Nakamura, Chie Sotozono, Tetsuro Oshika, Takeshi Morimoto, Kohji Nishida, Noriko Nishikawa, Young-Seok Song, Tomoki Tokutake, Yasuyo Nishi, Yuta Shigeno, Toshihide Kurihara, Kazuno Negishi, Kazuo Tsubota, Masafumi Ono, Tomoko Nakai, Donald Tan, Shiro Tanaka, and Shigeru Kinoshita).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
O. Hieda, Provision of drugs, translation and submission of this article (Eye-Lens Pte., Ltd); T. Hiraoka, None; T. Fujikado, None; S. Ishiko, None; S. Hasebe, None; H. Torii, None; H. Takahashi, None; S. Tanaka, Consulting fees (Daiichi-Sankyo, Satt, Welby, Eli Lilly), Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Bayer, Research Institute of Healthcare Data Science, Amgen); S. Kinoshita, Grants or contracts (Santen, Senju, HOYA), Consulting fees (Santen, Alcon, At Working), Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Santen, Senju, Alcon, Johnson & Johnson Vision, At Working).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Osamu Hieda
About this article
Cite this article
Hieda, O., Hiraoka, T., Fujikado, T. et al. Assessment of myopic rebound effect after discontinuation of treatment with 0.01% atropine eye drops in Japanese school-age children. Jpn J Ophthalmol 67, 602–611 (2023). https://doi.org/10.1007/s10384-023-01012-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-023-01012-8