Abstract
Background and aims
Perforation is a common complication during endoscopic resection (ER) of gastric gastrointestinal stromal tumors (gGISTs) associated with secondary infections, sepsis, hospitalization time and cost. However, the risk factors of perforation remain controversial. This study aimed to investigate the risk factors for perforation during ER of gGISTs.
Methods
This retrospective case–control study included consecutive patients with gGISTs who underwent ER between June 2009 and November 2021 at the Nan**g Drum Tower Hospital. Univariate and multivariate analyses were performed to investigate the risk factors for perforation. Sensitivity analyses with propensity scoring (PS) were performed to evaluate the stability of the independent effects.
Results
In total, 422 patients with gGISTs were included. The following factors were associated with perforation during ER: in the non-intraluminal growth patterns (all confounders adjusted odds ratio [aOR]: 5.39, 95% CI 2.99–9.72, P < 0.001), in the gastric fundus (aOR 2.25, 95% CI 1.40–3.60, P = 0.007), sized ≥ 2 cm (aOR 1.70, 95% CI 1.04–2.77, P = 0.035), in the lesser curvature (aOR 0.12, 95% CI 0.05–0.27, P < 0.001), and in the gastric cardia (aOR 0.13, 95% CI 0.04–0.50, P = 0.003). The PS analysis confirmed the stable independent effects of these identified risk factors.
Conclusions
ERs of gGISTs in non-intraluminal growth patterns, in the gastric fundus, and with larger tumor size were independent risk factors for perforation. While tumors in the lesser curvature or gastric cardia were independent protective factor for perforation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrointestinal stromal tumor (GIST) is the most prevalent type of mesenchymal tumors of the digestive tract, with 60–70% occurring in the stomach [1]. The annual incidence of malignant GISTs is relatively low (10 cases per million), but asymptomatic small GISTs (< 20 mm) can be detected in approximately 35% of patient autopsies [2]. Although the use of tyrosine kinase inhibitors has significantly improved the prognosis of gastric GISTs (gGISTs), surgical resection remains the main therapeutic choice [3, 4]. Endoscopic resection (ER) has recently been adopted as an alternative modality to laparoscopic wedge excision for removing small gGISTs (< 35 mm), because it is less invasive and has comparable recurrence, disease-free survival, and complication rates [5,6,7,8,9,10]. Moreover, ER has been recommended as an alternative to surveillance for treating gGISTs smaller than 20 mm [11].
Although tremendous progress has been made in endoscopic techniques, tumor recurrence and perforation remain as pivotal disadvantages. To reduce tumor recurrence, multiple new endoscopic techniques have been reported to achieve en bloc resection, and several stratification systems have been established to predict R1 resection for additional surgery [12,13,14]. Perforation is not rare in gGISTs who undergo ER (rates ranged from 6.7 to 62.5%), although most can be managed with endoscopic intervention or conservative therapy [7, 8, 15,16,17,18]. However, endoscopic perforation is closely related to secondary infections and sepsis, and was associated with longer hospitalization time and higher hospitalization cost [7, 8]. Therefore, it is crucial to identify the risk factors for perforation of gGISTs during ER.
A large lesion size and extraluminal growth are independent risk factors for perforation in patients with GISTs who underwent ER [19, 20]. Although gGISTs were included in these studies, the lack of confounders associated with the stomach, such as anatomical and circumferential locations of the tumor, limited the generalizability of the conclusions. Several studies found that lesions in the fundus had a higher perforation rate than those in other locations, but other studies found no significant difference in perforation rate between tumor locations [12, 21, 22]. In addition, the independent association between tumor location and perforation incidence has not been investigated.
This study aimed to investigate the independent risk factors associated with perforation in gGISTs treated with ER. Subgroup and multiple sensitivity analyses were performed to validate independent effects.
Methods
Study design and participants
This retrospective case–control study was conducted at the Nan**g Drum Tower Hospital (NDTH) and was approved by the Medical Ethics Committee of NDTH (approval no. 2022–110-01). The inclusion criteria were consecutive patients who underwent ER and were diagnosed with gGISTs by pathological and immunohistochemical examination between June 2009 and November 2021 in the NDTH. GISTs were diagnosed based on the immunohistochemical reactions of CD117, DOG-1 and CD34 [23]. The exclusion criteria were as follows: (1) patients with multiple synchronous gGISTs, gastric intraepithelial neoplasia or carcinomas; (2) patients with a history of subtotal gastrectomy; (3) patients who underwent incomplete resection; and (4) patients who underwent ER with intentional perforation. Intentional perforation was conducted by cap-assisted endoscopic full-thickness resection (EFR-C) according to Yang et al. [24], which was selectively carried out from May 2012 and indicated for gGISTs smaller than 1.5 cm, accounting for 29.3% (92/314) of all ≤ 1.5 cm cases.
ER procedures
ER was performed under sedation with intravenous propofol (1.5–2.5 mg/kg for induction and 4–12 mg/kg/h for maintenance) and midazolam (0.05 mg/kg) or general anesthesia with endotracheal intubation in the left decubitus position. All procedures were performed by 14 qualified endoscopists (Supplementary Table 1) using a single-channel endoscope (Q-260 J; Olympus) with a transparent cap (ND-201–11,802; Olympus). Endoscopic submucosal dissection (ESD) was attempted first in all cases according to ** the MP layer intact. For tumors with close connection to the serosal layer, Endoscopic subserosal dissection (ESSD) was attempted first during separation of the tumor from the serosal layer as we previously reported [26] (Fig. 1 b, Video 1): a subserosal injection is used to separate the tumor from the outer omentum layer while maintaining serosal layer integrity. The tumor is then gradually separated within the subserosal space, with the serosal layer remaining intact, eventually achieving complete resection. However, if ESD and ESSD fail to achieve complete resection while kee** the MP layer or serosal layer intact, Endoscopic Full-Thickness Resection (EFTR) is performed as a priority to ensure complete resection according to previous reports [13, 27] (Fig. 1c). EFTR involves a full-thickness resection of both the tumor and the surrounding stomach wall, including the entire MP and serosal layer. After EFTR, the gastric cavity is directly connected to the abdominal cavity, resulting in perforation in all cases. After dissection, the incision was closed using hemostatic clips (HX-610–90, HX-600–135, Olympus; ROCC-D-26–195-C, micro-tech, Nan**g, China).
Endoscopic resection methods for gastric gastrointestinal stromal tumors. a Endoscopic submucosal dissection (ESD): a-1 Marking, submucosa injection and circumferential incision; a-2 Expose the tumor, excavate and dissect from the muscularis propria; a-3 Remove the tumor with the muscularis propria layer intact; a-4 Closure of the incision; b: Endoscopic subserosal dissection (ESSD): b-1 Subserosal injection; b-2 Excavate the tumor with serosal layer intact; b-3 Compete serosal layer after dissection with good gas storage capacity of the stomach; b-4 Closure of the incision; c Endoscopic full-thickness resection (EFTR): c-1 Tumor with extensive connection with serosal layer; c-2 Full-thickness resection for complete resection; c-3 Full-thickness perforation after resection; c-4: Closure of the incision
Variables and outcomes
The primary outcome was perforation. Perforation was defined as direct endoscopic visualization of extra-gastric structures through an incomplete serosal layer (Intraoperative perforation) or radiographic evidence of pneumoperitoneum, and delayed perforation was defined as radiographic proved pneumoperitoneum without obvious intraoperative perforation [7]. En bloc resection was defined as the resection of a lesion with an intact capsule retrieved from the oral cavity. Complete resection was defined as tumors removed en bloc, with histopathological residual-negative margin (R0) [28]. The location of the gGIST lesion was classified into four circumferential locations (lesser curvature, anterior wall, greater curvature, and posterior wall) and five anatomical locations (cardia, fundus, body, angle, and antrum) [29, 30]. The tumor size was measured by assessment of the postoperative resected specimen in vitro. The tumor growth pattern was estimated mainly using CT images, whereas small gGISTs undetected on CT were estimated using endoscopic ultrasound [31]. If over 90% of the tumor body was bulging inside or outside the gastric luminal space, it was defined as either an intra- or extraluminal type respectively, whereas if 50–90% of the tumor body was bulging inside or outside the gastric luminal space it was defined as either an intra- or extraluminal-dominant type respectively (Fig. 2) [19]. Malignancy potential was classified according to the modified National Institutes of Health (NIH) classification system of 2008 [32]. The operators were classified into two groups according to their experience in ER of upper digestive tumors (< or ≥ 2000 cases). The learning curve effect was evaluated by dividing the first half of the ER procedures by the second half of each operator [33]. Adverse events were classified according to the Clavien–Dindo Grade [34]. Recurrence was defined as the detection of tumor by upper endoscopy or abdominal CT scan during follow-up in the primary resection site (local) or distance [7]. Follow-up was performed 6 months after ER operation to evaluate local recurrence by upper endoscopy and annually thereafter by a surveillance endoscopy and abdominal CT scan [35]. The last observation was in 21 May 2022.
Computed tomography (CT) evaluation of gastric gastrointestinal stromal tumor (gGIST) growth pattern (white arrow). a Intraluminal growth: CT showed a gGIST with over 90% of tumor bulging into the intraluminal space; b Intraluminal-dominant growth: CT showed a gGIST with 2/3 of tumor bulging into the intraluminal space; c Extraluminal-dominant growth: CT showed a gGIST with 2/3 of tumor bulging into the extraluminal space; d Extraluminal growth: CT showed a gGIST mainly bulging into the extraluminal space
Statistical analysis
Data were categorized and described as frequencies (%) and compared between groups using the chi-squared test or Fisher’s exact test. Univariate logistic regression was performed to investigate the effect size of the potential risk factors for perforation, and results were presented as odds ratios (ORs) and 95% confidence intervals (CIs). Based on the univariate analysis, we combined groups with similar effect values when performing multivariate analyses. Significant risk factors (p < 0.05) in univariate analysis (anatomical location, circumferential location, growth pattern, and tumor size) were entered into a multivariate logistic regression model. The independent effect of the risk factors was assessed using two multiple logistic regression models, adjusted for selected confounding variables. Confounders adjusted in Model I were selected based on their associations with the outcomes of a change in effect estimated around > 10% (data not shown). In Model II, all potential covariates were included in the adjustment. The propensity score (PS) was applied as a sensitivity analysis to account for selection bias and ensure the stability of the independent effect, including inverse probability of treatment weighing (IPTW), propensity-score matching (PSM), and adjustment for propensity score [36, 37]. In the IPTW analysis, each patient was weighted by inverse probability to calculate the stabilized IPTW weight from all confounders. PSM analysis was used to calculate the propensity scores for each patient using a multivariable logistic regression model that included all potential covariates. The nearest-neighbor method was used to create a matched control group at a 1:1 ratio. Statistical significance was set at P < 0.05 (two-sided). All analyses were performed using EmpowerStats (https://www.empowerstats.com) and the R statistical software (version 4.1.2; R Project for Statistical Computing).
Results
Baseline and clinicopathological characteristics
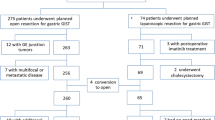
In total, 422 patients with 422 lesions were included in the final analysis including 169 lesions with perforation (163 intraoperative perforation and 6 delayed perforation, shown in Supplementary Table 2) and 253 controls without perforation (Fig. 3). The baseline and clinicopathological characteristics are shown in Table 1 and the proportion of each single item is shown in Fig. 4. Among all patients, 42.2% were male and 53.3% were aged ≥ 60.0 years. No statistically significant differences were observed in age (P = 0.413) or sex (P = 0.954) between the cases and controls.
Compared with controls, fewer lesions in perforation group were located in the lesser curvature (5.3% vs. 24.1%, P < 0.001) and gastric cardia (1.8 vs. 9.1%, P < 0.001), whereas more lesions were located in the gastric fundus (60.4 vs. 41.9%, P < 0.001). More tumors in the perforation group were > 2 cm in size (42.6 vs. 30.8%, P = 0.013). The vast majority of lesions without perforation had an intraluminal growth pattern (83.8%); the latter was less prevalent in perforated lesions (61.5%, P < 0.001). The risk categories of the modified NIH classification for the cases and controls were as follows: very low (56.2 vs. 66.4%), low (36.2 vs. 26.5%), intermediate (5.9 vs. 4.7%), and high (1.8 vs. 2.4%). The experience (≥ 2000 cases: 43.8 vs. 41.5%, P = 0.642) and learning curvature (Second 50%: 56.2% vs. 47.0%, P = 0.065) of the operators were comparable between cases and controls.
Comparison of clinical outcomes
The clinical outcomes of ER with and without perforation were compared and shown in Table 2. Compared with controls, cases with perforation during ER were associated with a higher rate of sepsis (OR 3.67, 95% CI 1.40–9.63), a longer time until start of oral intake (b: 0.62 (days), 95% CI 0.40–0.84), longer postoperative hospital stays (b 1.13 (days), 95% CI 0.54–1.72) and higher total cost (b: 255.84 ($), 95% CI 114.20–397.48) in multivariate analysis after adjusting for all confounders including age, sex, anatomical location, circumferential location, tumor size, growth pattern, operator, and operator learning curve. During a follow-up with mean time of 4.3 years, patients underwent ER without perforation showed no recurrence. While among patients with perforation during a mean follow-up of 3.7 years, one patient was detected recurrence in pancreas 6.4 years after first resection. The primary tumor size was 1.5 cm with a mitotic index of 4.5/50 HPF.
Risk factors for ER perforation
Then, a univariable logistic regression analysis was performed to evaluate the risk factors for perforation. The results showed that tumors in non-intraluminal growth patterns (OR, 3.23; 95% CI, 2.05–5.10), located in the fundus (OR 2.11; 95% CI, 1.42–3.14), and sized ≥ 2 cm (OR 1.67; 95% CI 1.11–2.50) were associated with higher perforation risk. Tumors located in the lesser curvature (OR 0.18, 95% CI 0.09–0.37) or in cardia (OR 0.18, 95% CI 0.05–0.61) were associated with lower risk of perforation (Table 3).
The independent effect for each of these five factors remained significant when assessed by binary multivariable logistic regression analysis. Compared with cases located in lesser curvature, cases in anterior wall (OR 11.84; 95% CI 4.64–30.21), in greater curvature (OR 10.38; 95% CI 4.24–25.43), and in posterior wall (OR, 5.22; 95% CI, 2.03–13.46) were associated with a higher perforation risk. Compared with locating in gastric body, higher perforation risk was identified for locating in fundus (OR 1.78; 95% CI 1.07–2.94), and lower risk in cardia (OR, 0.21; 95% CI, 0.05–0.80). Tumor larger than 2 cm was also associated with a higher perforation risk (OR 1.68, 95%CI 1.05–2.68). Moreover, compared with cases in intraluminal pattern, cases in intraluminal-dominant pattern (OR 4.21; 95% CI 2.19–8.09), in extraluminal-dominant pattern (OR 11.95; 95% CI 3.56–40.05), and in extraluminal pattern (OR 9.13; 95% CI 2.28–36.61) were all associated with higher perforation risk.
Sensitivity analysis
Further sensitivity analyses were performed to evaluate the independent effects of these identified factors associated with perforation (Fig. 5). Baseline characteristics before and after IPTW/PSM were shown in Supplemental Table 3–12. Cases located in the lesser curvature were strongly associated with a lower risk of perforation in multiple models (Model I adjusted for growth pattern: OR 0.12; 95% CI 0.05–0.26; Model II adjusted for all: OR 0.12; 95% CI 0.05–0.27; IPTW: OR, 0.20; 95% CI 0.12–0.31; PSM: OR, 0.24; 95%, CI 0.10–0.61; PS adjusted: OR, 0.17; 95% CI, 0.08–0.36). Cases located in the gastric cardia were also identified as a protective factor for perforation (Model I adjusted for circumferential location: OR 0.15; 95% CI, 0.04–0.52; Model II adjusted for all: OR 0.13; 95% CI, 0.04–0.50; IPTW: OR 0.27; 95% CI, 0.17–0.42; PSM: OR 0.35; 95% CI 0.08–1.56; PS adjusted: OR 0.18; 95% CI 0.05–0.60). Cases located in the gastric fundus were strongly associated with a higher risk of perforation (Model I adjusted for age, circumferential location, and growth pattern: OR 2.21; 95% CI 1.39–3.49; Model II adjusted for all: OR 2.25; 95% CI 1.40–3.60; IPTW: OR 1.96; 95% CI 1.32–2.92; PSM: OR 2.33; 95% CI 1.42–3.84; PS adjusted, OR 1.92, 95% CI 1.26–2.92). Meanwhile, lesions with a larger size (> 2 cm) were at a higher risk of perforation (Model I adjusted for age, circumferential location, and growth pattern: OR, 1.82; 95% CI 1.14–2.90; Model II adjusted for all: OR 1.70; 95% CI 1.04–2.77; IPTW: OR 1.79; 95% CI 1.21–2.64; PSM: OR 1.54; 95% CI 0.91–2.60; PS adjusted: OR, 1.52; 95% CI, 0.98–2.34). Moreover, the presence of a non-intraluminal growth pattern was identified as a strong independent risk factor for perforation (Model I adjusted for anatomic and circumferential location: OR 6.06; 95% CI 3.40–10.81; Model II adjusted for all: OR 5.39; 95% CI 2.99–9.72; IPTW: OR 4.29; 95% CI 2.85–6.45; PSM: OR 5.23; 95% CI 2.81–9.73; PS adjusted: OR 3.82; 95% CI 2.33–6.25).
The effect size of risk factors for perforation in crude univariable, multivariable, and propensity-score analyses. a Adjusts for tumor growth pattern; b Adjusts for all other varieties; c Adjusts for circumferential location; d Adjusts for age, circumferential location and growth pattern; e Adjusts for anatomic location and circumferential location
Discussion
In this retrospective case–control study, we evaluated the risk factors associated with perforation of gGISTs during ER. The results showed that cases in a non-intraluminal growth pattern (all confounders adjusted odds ratio [aOR], 5.39; 95% CI 2.99–9.72), located in the gastric fundus (aOR, 2.25; 95% CI 1.40–3.60), and sized ≥ 2 cm (aOR 1.70; 95% CI 1.04–2.77) were independent risk factors, while those located in the lesser curvature (aOR 0.12; 95% CI 0.05–0.27) or gastric cardia (aOR 0.13; 95% CI 0.04–0.50) were protective factors for perforation. Multiple sensitivity analyses validated the stable effect value of these factors. To the best of our knowledge, our study included the largest sample size and number of variables to evaluate independent risk factors for perforation of gGISTs during ER. This study provides evidence for more precise endoscopic management strategies to minimize perforation of gGISTs under ER. Meanwhile, by identifying the cases with high risk of perforation, more aggressive interventions are optional including gastric lavage with a dilute antibiotic solution to reduce secondary infections and other related adverse events [38].
According to the latest consensus, endoscopic resection is suggested to be an optional therapeutic modality for small gGISTs, and the object of operation is to achieve R0 resection and avoid tumor rupture as far as possible, eventually ensuring a complete resection [4, 35, 39]. Tumor rupture and R1 resection were associate with higher risk factor of peritoneal seeding, recurrence and a poor prognosis during resection of gastric GISTs [40, 41]. Therefore, achieving complete resection was the precondition of ER for gGISTs. For the patients received incomplete resection, the eventual perforation status of the additional treatment was unmeasurable. Because of the adverse events that may be caused by incomplete resection, optimizing the operation method and reducing perforation rate became nonsense in such cases. Meanwhile, multiple previous studies have reported relatively high complete resection rate ranging from 88 to 100% [9, 42,43,44]. Under our inclusive and exclusive criteria, the complete resection rate achieved 90.0% (422/469). The relatively high complete resection rate made us exclude the R1 resection cases to focus on the majority population and reduce the bias. Therefore, under such inclusion and exclusion criteria we believed that the result of our study was well generalized and could provide evidence for clinical application value.
Iatrogenic perforation is a frequent complication in gGISTs under ER, while perforation during operation was reported associated with secondary infections and sepsis [7, 8]. In this present study a higher incidence of sepsis, a longer time until start of oral intake, longer postoperative hospital stays and higher total cost were identified in patients underwent ER with perforation. Thus, it is crucial to identify the risk factors for perioperative perforation of gGISTs during ER. According to previous studies, the perforation rate ranges 6.7–62.5% [7, 8, 15,16,17,18], and varies according to the tumor location, size, and growth pattern [19,20,21, 45]. Zhang et al. reported that, compared with the body (11.5%), the fundus of the stomach was the site of more perforations (21.5%) of gastric subepithelial tumors during endoscopic excavation. He et al. [45] also revealed that the perforation rate of gastric subepithelial tumors in the fundus (23.4%) was higher than that in the body (4.7%). Kim et al. [7] reported 6 cases of macroperforation in ER group all locating in the fundus or body. However, several other reports observed that the perforation rate of lesions was higher in the body [29, 46]. These conflicting results may be due to two reasons: (1) the heterogeneity of the clinical features of the included patients and endoscopists with varied expertise, and (2) that all previous studies only compared the perforation rate in different groups and did not adjust for potential confounders to calculate the independent effects of tumor location on perforation. In this study, we also found that lesions in the fundus showed a remarkably higher perforation risk than those in other locations, and lesions in the cardia exhibited a lower perforation risk than those in other sites. We collected potential confounders related to the lesions and endoscopists and used comprehensive methods to adjust for these confounders. We found that lesions in the fundus were associated with higher perforation risk (aOR 2.25; 95% CI 1.40–3.60), and those in the cardia were associated with fewer perforations (aOR 0.13; 95% CI 0.04–0.50) via regression analysis, propensity score, and subgroup analysis. The varied perforation risks in different anatomic locations may be a result of the different thicknesses of the gastric walls at these sites.
Multiple studies have explored the association between anatomic location and perforation rate and found that locating in upper third of stomach, especially in gastric fundus, was associated with higher perforation rate [30, 47, 48]. The main reason may be the difficulty in manipulating the endoscope and the thickness of the gastric wall in fundus was thinner than in other locations [49, 50]. Similar result was also confirmed in our study. However, few studies have evaluated the role of the circumferential location of gGISTs. Kim et al. [29] reported that early gastric cancer in the lesser curvature showed a lower perforation rate (1.5%) than other locations (2.0–5.0%), but no independent association was observed. Suzuki et al. [51] found that early gastric cancer in the greater curvature manifested a higher perforation rate (0.4%) than in other parts (0.1%), although multivariate analysis showed no significant effects. Notably, Ojima et al. [52] reported that gastric epithelial lesions in the greater curvature were an independent risk factor associated with perforation (OR 7.04; 95% CI 3.13–15.80), since the greater curvature had limited visual field and blood retention caused by gravity. Here, we found that gGISTs in the lesser curvature showed a significantly lower perforation risk than those in other locations, which has not been reported before. Multivariate analysis and several sensitivity analyses revealed that lesions in the lesser curvature were an independent protective factor (aOR 0.12; 95% CI 0.05–0.27) for perforation. The lesser curvature of the stomach is where the greater and lesser omentum converge, which provides space for the connective tissue envelo** the serosal layer. According to our previous reports, endoscopic subserosal dissection (ESSD) of submucosal tumors (SMTs) performed in this space is feasible and safe while maintaining the integrity of the serosa and reducing the risk of adverse events [26]. Therefore, gGISTs in the lesser curvature were easier to lift and separate due to the existence of the space for the connective tissue, thus reducing the occurrence of perforation.
We also investigated the effects of tumor size and growth pattern on perforation of gGISTs during ER. Meng et al. [46]. found that the perforation rate of gGIST with a large tumor size (> 20 mm, 37.5%) was higher than that of small tumors (≤ 20 mm, 9.1%), but the independent risk effect was not calculated. Ye et al. [19] identified that larger tumor size (OR 1.54; 95% CI 1.07–2.24), extraluminal growth (OR 1.77; 95% CI 1.01–3.11), and extensive connection to the MP layer (OR 10.47; 95% CI 5.00–21.93) were independent risk factors for perioperative perforation of upper gastrointestinal SMTs, including 250 gGISTs. Ye and Xu et al. [12, 20] reported similar results for SMTs treated with ESTR and found that lesions with larger tumor size and extraluminal growth showed a higher perforation rate. However, all these studies included other histological types of SMT, while GISTs tended to have more perforations compared with other histological types [12, 21, 53]. In the present study, size ≥ 2 cm (aOR 1.70; 95% CI 1.04–2.77), and non-intraluminal growth pattern (aOR 5.39; 95% CI 2.99–9.72) were associated with higher perforation risk of ER for gGISTs, consistent with these previous studies on SMT [12, 53]. Gastric GISTs with non-intraluminal growth patterns and larger tumor sizes are often difficult to resect endoscopically [14]. Although several novel methods have been established to successfully resect these gGISTs en bloc, perforation is difficult to avoid due to the limited surgical space and relatively thin gastric wall [31, 54]. We noticed that experienced endoscopists could achieve a lower perforation rate in non-intraluminal gGISTs (OR 0.37; 95% CI 0.17–0.84; data not shown). This indicates that these lesions should be managed by experienced endoscopists to reduce complications.
Despite the large sample size and sufficient sensitivity analysis, this study had some limitations. First, because of the nature of this single-center retrospective study, selection bias could exist. Thus, we performed multiple sensitivity analyses of the PS to minimize bias and evaluate the dependent effect of each risk factor. Second, because intentional perforation is needed in certain cases to achieve complete resection, it is challenging to distinguish the two types of perforation (accidental perforation during ESD and needed perforation performed by EFTR) for the nature of our retrospective study. To minimize bias, we excluded all cases that underwent EFR-C with intentional perforation [24]. Third, as we excluded the cases with incomplete resection to reduce the bias of perforation, the results were not applicable to incomplete resections and the risk factors associated with incomplete resection were not evaluated. Future studies will focus more on incomplete resection. Finally, the sample size and events of the extraluminal group were insufficient to perform a detailed subgroup analysis; thus, we combined the groups into non-intraluminal growth patterns. Nevertheless, our study provides sufficient information to establish a more precise endoscopic management strategy for gGISTs.
Conclusions
This study identified that tumors in non-intraluminal growth patterns, with a larger size, and located in the fundus are independent risk factors, while located in the lesser curvature or cardia are independent protective factors for perforation of gGISTs during ER. This study provides evidence for more precise endoscopic management strategies to minimize perforation of gGISTs under ER.
Data availability
All researchers can submit a request to acquire the anonymized data presented in this manuscript from the corresponding author (Lei Wang). The decision to disclose the data will be made by the corresponding author. The data disclosure can be requested for 36 months from the article publication.
Abbreviations
- CI:
-
Confidence interval
- EFTR:
-
Endoscopic full–thickness resection
- ER:
-
Endoscopic resection
- ESD:
-
Endoscopic submucosal dissection
- EU:
-
Endoscopic ultrasound
- GIST:
-
Gastrointestinal stromal tumor
- IPTW:
-
Inverse probability of treatment weighting
- MP:
-
Muscularis propria
- NIH:
-
National Institutes of Health
- PS:
-
Propensity score
- PSM:
-
Propensity score matching
- SMTs:
-
Submucosal tumors
References
Miettinen M, El-Rifai W, HLS L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478–83.
Kawanowa K, Sakuma Y, Sakurai S, Hishima T, Iwasaki Y, Saito K, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol. 2006;37(12):1527–35.
Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, et al. NCCN task force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010. https://doi.org/10.6004/jnccn.2010.0116.
von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN, et al. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(5):536–63.
Joo MK, Park JJ, Kim H, Koh JS, Lee BJ, Chun HJ, et al. Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointest Endosc. 2016;83(2):318–26.
Meng Y, Li W, Han L, Zhang Q, Gong W, Cai J, et al. Long-term outcomes of endoscopic submucosal dissection versus laparoscopic resection for gastric stromal tumors less than 2 cm. J Gastroenterol Hepatol. 2017;32(10):1693–7.
Kim GH, Choi KD, Gong CS, Lee IS, Park YS, Han M, et al. Comparison of the treatment outcomes of endoscopic and surgical resection of GI stromal tumors in the stomach: a propensity score-matched case-control study. Gastrointest Endosc. 2020;91(3):527–36.
Dong X, Chen W, Cui Z, Chen T, Liu X, Chen D, et al. Laparoscopic resection is better than endoscopic dissection for gastric gastrointestinal stromal tumor between 2 and 5 cm in size: a case-matched study in a gastrointestinal center. Surg Endosc. 2020;34(11):5098–106.
Zhu H, Zhao S, Jiao R, Zhou J, Zhang C, Miao L. Comparison of endoscopic versus laparoscopic resection for gastric gastrointestinal stromal tumors: a preliminary meta-analysis. J Gastroenterol Hepatol. 2020;35(11):1858–68.
Liu Z, Zeng Z, Ouyang S, Zhang Z, Sun J, Wang X, et al. Comparison among endoscopic, laparoscopic, and open resection for relatively small gastric gastrointestinal stromal tumors (<5 cm): a Bayesian network meta-analysis. Front Oncol. 2021;11: 672364.
Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN clinical practice guidelines for diagnosis treatment and follow-up. Ann Oncol. 2018;29:iV51-iv67.
Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X, Chen JY. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc. 2014;28(2):524–30.
Wang L, Ren W, Fan CQ, Li YH, Zhang X, Yu J, et al. Full-thickness endoscopic resection of nonintracavitary gastric stromal tumors: a novel approach. Surg Endosc. 2011;25(2):641–7.
Su W, Wang M, Zhang D, Zhu Y, Lv M, Zhu L, et al. Predictors of the difficulty for endoscopic resection of gastric gastrointestinal stromal tumor and follow-up data. J Gastroenterol Hepatol. 2022;37(1):48–55.
Yu C, Liao G, Fan C, Yu J, Nie X, Yang S, et al. Long-term outcomes of endoscopic resection of gastric GISTs. Surg Endosc. 2017;31(11):4799–804.
Huang LY, Cui J, Liu YX, Wu CR, Yi DL. Endoscopic therapy for gastric stromal tumors originating from the muscularis propria. World J Gastroenterol. 2012;18(26):3465–71.
Chen Q, Yu M, Lei Y, Zhong C, Liu Z, Zhou X, et al. Efficacy and safety of endoscopic submucosal dissection for large gastric stromal tumors. Clin Res Hepatol Gastroenterol. 2020;44(1):90–100.
Shichijo S, Uedo N, Yanagimoto Y, Yamamoto K, Kono M, Fukuda H, et al. Endoscopic full-thickness resection of gastric gastrointestinal stromal tumor: a Japanese case series. Ann Gastroenterol. 2019;32(6):593–9.
Ye LP, Zhang Y, Luo DH, Mao XL, Zheng HH, Zhou XB, et al. Safety of endoscopic resection for upper gastrointestinal Subepithelial tumors originating from the muscularis Propria layer: an analysis of 733 tumors. Am J Gastroenterol. 2016;111(6):788–96.
Chen T, Zhou PH, Chu Y, Zhang YQ, Chen WF, Ji Y, et al. Long-term outcomes of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Ann Surg. 2017;265(2):363–9.
Zhang Y, Ye LP, Zhou XB, Mao XL, Zhu LH, He BL, et al. Safety and efficacy of endoscopic excavation for gastric subepithelial tumors originating from the muscularis propria layer: results from a large study in China. J Clin Gastroenterol. 2013;47(8):689–94.
Bialek A, Wiechowska-Kozlowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpinska K, et al. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc. 2012;75(2):276–86.
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–8.
Yang J, Ni M, Jiang J, Ren X, Zhu T, Cao S, et al. Comparison of endoscopic full-thickness resection and cap-assisted endoscopic full-thickness resection in the treatment of small (</=1.5 cm) gastric GI stromal tumors. Gastrointest Endosc. 2021. https://doi.org/10.1016/j.gie.2021.10.026.
An W, Sun PB, Gao J, Jiang F, Liu F, Chen J, et al. Endoscopic submucosal dissection for gastric gastrointestinal stromal tumors: a retrospective cohort study. Surg Endosc. 2017;31(11):4522–31.
Liu F, Zhang S, Ren W, Yang T, Lv Y, Ling T, et al. The fourth space surgery: endoscopic subserosal dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc. 2018;32(5):2575–82.
Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25(9):2926–31.
Shi Q, Zhong YS, Yao LQ, Zhou PH, Xu MD, Wang P. Endoscopic submucosal dissection for treatment of esophageal submucosal tumors originating from the muscularis propria layer. Gastrointest Endosc. 2011;74(6):1194–200.
Kim M, Jeon SW, Cho KB, Park KS, Kim ES, Park CK, et al. Predictive risk factors of perforation in gastric endoscopic submucosal dissection for early gastric cancer: a large, multicenter study. Surg Endosc. 2013;27(4):1372–8.
Ohta T, Ishihara R, Uedo N, Takeuchi Y, Nagai K, Matsui F, et al. Factors predicting perforation during endoscopic submucosal dissection for gastric cancer. Gastrointest Endosc. 2012;75(6):1159–65.
Cai MY, Zhu BQ, Xu MD, Qin WZ, Zhang YQ, Chen WF, et al. Submucosal tunnel endoscopic resection for extraluminal tumors: a novel endoscopic method for en bloc resection of predominant extraluminal growing subepithelial tumors or extra-gastrointestinal tumors (with videos). Gastrointest Endosc. 2018;88(1):160–7.
Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39(10):1411–9.
Bronswijk M, Vanella G, van Malenstein H, Laleman W, Jaekers J, Topal B, et al. Laparoscopic versus EUS-guided gastroenterostomy for gastric outlet obstruction: an international multicenter propensity score-matched comparison (with video). Gastrointest Endosc. 2021;94(3):526–33.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96.
Koo DH, Ryu MH, Kim KM, Yang HK, Sawaki A, Hirota S, et al. Asian consensus guidelines for the diagnosis and management of gastrointestinal stromal tumor. Cancer Res Treat. 2016;48(4):1155–66.
Regal RR, Hook EB. Marginal versus conditional versus “structural source” models: a rationale for an alternative to log-linear methods for capture-recapture estimates. Stat Med. 1998;17(1):69–74.
Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163(3):262–70.
Rajan E, Song WK, LM. Endoscopic full thickness resection. Gastroenterology. 2018;154(7):1925–37.
Li J, Ye Y, Wang J, Zhang B, Qin S, Shi Y, et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res. 2017;29(4):281–93.
Hohenberger P, Ronellenfitsch U, Oladeji O, Pink D, Strobel P, Wardelmann E, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg. 2010;97(12):1854–9.
Yanagimoto Y, Takahashi T, Muguruma K, Toyokawa T, Kusanagi H, Omori T, et al. Re-appraisal of risk classifications for primary gastrointestinal stromal tumors (GISTs) after complete resection: indications for adjuvant therapy. Gastric Cancer. 2015;18(2):426–33.
Joo MK, Park JJ, Lee YH, Lee BJ, Kim SM, Kim WS, et al. Clinical Efficacy and safety of endoscopic treatment of gastrointestinal stromal tumors in the stomach. Gut Liver. 2023. https://doi.org/10.5009/gnl210454.
Huang L, Jia YX, Lyu B, Meng LN, ** HF. Effects of endoscopic submucosal excavation with non-submucosal injection on stromal tumors in stomach. Front Oncol. 2022;12: 792445.
Cho J, Han J, Choi M, Song J, Yang M, Lee Y. Correlation between endoscopic resection outcomes and endosonographic findings in gastric tumors with muscularis propria origin. Medicine (Baltimore). 2022;101(32): e29947.
He Z, Sun C, Wang J, Zheng Z, Yu Q, Wang T, et al. Efficacy and safety of endoscopic submucosal dissection in treating gastric subepithelial tumors originating in the muscularis propria layer: a single-center study of 144 cases. Scand J Gastroenterol. 2013;48(12):1466–73.
Meng FS, Zhang ZH, Shan GD, Chen YP, Ji F. Endoscopic submucosal dissection for the treatment of large gastric submucosal tumors originating from the muscularis propria layer: a single center study. Z Gastroenterol. 2015;53(7):655–9.
Yoon JY, Shim CN, Chung SH, Park W, Chung H, Lee H, et al. Impact of tumor location on clinical outcomes of gastric endoscopic submucosal dissection. World J Gastroenterol. 2014;20(26):8631–7.
Yoo JH, Shin SJ, Lee KM, Choi JM, Wi JO, Kim DH, et al. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: emphasis on perforation type. Surg Endosc. 2012;26(9):2456–64.
van Rutte PW, Naagen BJ, Spek M, Jakimowicz JJ, Nienhuijs SW. Gastric wall thickness in sleeve gastrectomy patients: thickness variation of the gastric wall. Surg Technol Int. 2015;27:123–8.
Zhao J, Liao D, Chen P, Kunwald P, Gregersen H. Stomach stress and strain depend on location, direction and the layered structure. J Biomech. 2008;41(16):3441–7.
Suzuki H, Oda I, Sekiguchi M, Abe S, Nonaka S, Yoshinaga S, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol. 2015;21(44):12635–43.
Ojima T, Takifuji K, Nakamura M, Iwahashi M, Nakamori M, Katsuda M, et al. Complications of endoscopic submucosal dissection for gastric noninvasive neoplasia: an analysis of 647 lesions. Surg Laparosc Endosc Percutan Tech. 2014;24(4):370–4.
Jeong ID, Jung SW, Bang SJ, Shin JW, Park NH, Kim DH. Endoscopic enucleation for gastric subepithelial tumors originating in the muscularis propria layer. Surg Endosc. 2011;25(2):468–74.
Lu J, Jiao T, Li Y, Zheng M, Lu X. Facilitating retroflexed endoscopic full-thickness resection through loop-mediated or rope-mediated countertraction (with videos). Gastrointest Endosc. 2016;83(1):223–8.
Acknowledgements
We thank Nurse Yufang Teng and Qin Yin for regular follow-up management of patients who underwent ER for gGISTs.
Funding
This study was funded by the China Postdoctoral Science Foundation (2022M721571), Jiangsu Provincial Health Commission (M20200034), and the key project of medical science and technology development of Nan**g Municipal Health Commission (ZKX21032).
Author information
Authors and Affiliations
Contributions
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. Conception and design: MN, LW; Development of methodology: DT, WR; Acquisition of data: RM, JY, PY; Analysis and interpretation of data: XD, GX, YL, HY, MC; Writing, review, and/or revision of the manuscript: MN, DT, WR; Study supervision: GX, YL, HY, MC, LW.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 173836 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ni, M., Tang, D., Ren, W. et al. Risk factors of perforation in gastric stromal tumors during endoscopic resection: a retrospective case–control study. Gastric Cancer 26, 590–603 (2023). https://doi.org/10.1007/s10120-023-01391-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-023-01391-4