Abstract
Background
The Korea National Cancer Screening Program (KNCSP) offers upper endoscopy or upper gastrointestinal series (UGIS) biannually for people aged ≥ 40 years. This study aimed to assess the effect of negative screening results on the incidence of and mortality from upper gastrointestinal (GI) cancer.
Methods
A population-based retrospective cohort of 15,850,288 men and women was constructed using data from 3 national databases. The participants were followed until the end of 2017 for data on cancer incidence and in 2019 for data on the vital status. Cox proportional hazard model with time-varying exposure was used to assess the association.
Results
By the end of the follow-up period, 230,783 upper GI cancer cases and 99,348 upper GI cancer deaths were recorded. Negative gastric cancer screening was significantly associated with a lower risk of upper GI cancer in both UGIS (adjusted hazard ratio [aHR] = 0.81, 95% CI = 0.80–0.82) and upper endoscopy (aHR = 0.67, 95% CI = 0.67–0.68) groups. The HRs for upper GI mortality were 0.55 (95% CI = 0.54–0.56) and 0.21 (95% CI = 0.21–0.22) for the UGIS and upper endoscopy groups, respectively. The most significant reductions in the risk of upper GI cancer (UGIS: aHR = 0.76, 95% CI = 0.74–0.77; upper endoscopy: aHR = 0.60, 95% CI = 0.59–0.61) and death (UGIS: aHR = 0.54, 95% CI = 0.52–0.55; upper endoscopy: aHR = 0.19, 95% CI = 0.19–0.20) were observed among individual aged 60–69 years.
Conclusion
Negative screening cases, especially in upper endoscopy of the KNCSP, were associated with an overall reduction in the risk of and mortality from upper GI cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrointestinal (GI) malignancies are the most common cancers diagnosed worldwide, which are responsible for approximately a quarter of incident cancer cases and one-third of cancer deaths [1]. The most common types of GI cancers are colorectal, gastric, liver, and esophageal cancer [1]. Although GI cancers share some risk factors, their causes, and epidemiological characteristics vary between cancer sites and geographical regions [2,3,4]. For example, gastric cancer only accounted for 5.6% of new cancer cases in all countries; this figure was about 10.9% in the Eastern Asia region in 2020 [5]. Consequently, some Eastern Asia countries, such as South Korea and Japan, introduced gastric cancer screening programs to tackle the disease burden, using evidence from observational studies [6,7,8]. Several studies have investigated the effect of this approach on the incidence and mortality rates of cancer in Asia [9,10,11,12].
In 2018, cancer became the most common cause of death, accounting for 26.5% of all deaths in South Korea [13]. GI cancer has been the most frequently diagnosed cancer in recent years, and gastric cancer alone contributed to approximately 12% of the cases (29,279 cases) [13]. Since 2002, the Korea National Cancer Screening Program (KNCSP) has been providing either upper endoscopy or upper gastrointestinal series (UGIS) biannually for those aged 40 years or older. As the primary purpose of KNCSP is to reduce the burden of gastric cancer, previous studies examined the effect of screening on gastric cancer mortality and survival [9,10,11, 14].
According to the quality control protocol of the KNCSP, both upper endoscopy and UGIS are recommended to examine for the entire upper GI tract from the esophagus to the duodenum. Therefore, gastric cancer screening is expected to help reduce the incidence of and mortality from upper digestive tract cancer. In particular, upper endoscopy is expected to be effective in reducing the risk of upper GI cancer by detecting upper digestive diseases or precancerous lesions as well as gastric cancer [15]. However, to the best of our knowledge, the effect of the gastric cancer screening program on the overall upper GI cancer burden has not yet been evaluated. Therefore, we conducted this study to assess the effect of negative screening cases on the incidence of and mortality from overall upper GI cancer in the target population of the KNCSP.
Methods
Study population
Our study used data from the KNCSP, an active database covering eligible cancer screening populations across South Korea. The database contains sociodemographic information about the target population and screening-related variables, including screening tests, dates, and results. For the current study, the baseline population included individuals aged 40 years or older who were invited to undergo gastric cancer screening in the KNCSP 2002 and 2003. Then, we linked the KNCSP to the Korean Central Cancer Registry (KCCR) database and the Death Certificate from Statistic Korea, which cover more than 95% and 99% of new cancer cases and deaths in South Korea, respectively [13]. To obtain the cancer-free cohort, we excluded people who died before their first screening invitation (n = 7995) and those with a history of cancer (n = 326,360). Then, people aged 80 (n = 748,758) and who had incomplete records (n = 119) were excluded from the study population. As the main aim of this study was to assess the risk of upper GI cancer and death in those with negative screening results, we excluded all the screening-detected GI cancer cases from 2002 to 2017 (n = 101,063). Finally, 15,850,288 men and women were followed until December 31, 2017, for data on cancer incidence and until December 31, 2019, for data on the vital status (Fig. 1).
Written informed consent was obtained from the participants in the KNCSP database for the collection of their screening results. The requirement for informed consent for the use of the collected data in the current study was waived owing to the use of de-identified data. The current study was approved by the Institutional Review Board of the National Cancer Center, Korea (No. NCCNCS08129).
Measurements
Exposure
In KNCSP, people were free to choose either upper endoscopy or upper gastrointestinal series (UGIS), an X-ray examination of the GI tract after they drank a liquid containing barium sulfate, for gastric cancer screening. In the case of UGIS examination, if suspicious findings were observed in UGIS, upper endoscopy was performed through KNCSP. If abnormal tissue was found during the upper endoscopic procedure, a biopsy could also be performed. According to the KNSCP protocol, both upper endoscopy and UGIS are recommended to examine the entire upper GI tract from the esophagus to the duodenum. Screening results for both screening methods were reported either as negative, peptic ulcer, benign tumor, possible gastric cancer, early gastric cancer, advanced gastric cancer, or other (e.g., esophageal/gastric varices, esophagitis, esophageal submucosal tumor, esophageal cancer, duodenal ulcer, duodenal cancer, duodenal submucosal tumor, etc.). During the upper endoscopy procedure, a biopsy could also be performed if any abnormal tissue (recorded as possible gastric cancer, early gastric cancer, advanced gastric cancer, or others where the physician deemed it necessary) was detected. In the case of those who chose UGIS, if suspicious findings were observed in UGIS, the patient was referred for upper endoscopy with biopsy for the final laboratory confirmation.
In this study, “negative screening cases” was defined as people who underwent gastric cancer screening through KNCSP and had no diagnosis of gastric cancer or other upper GI cancers within 1 year from the date of screening was made. We used 1 year as the maximum time for all follow-up procedures in the KNCSP [16]. Therefore, the term “negative screening case” in the current study indicates no GI cancer including gastric cancer at the time of screening. To estimate the effect of screening modality, the negative cases were further classified into upper endoscopy-negative, and UGIS-negative (upper endoscopy and UGIS in short) based on the screening test they underwent. The individuals with no screening record in our database were classified as “never-screened.”
The observational period of screening exposure was from January 1, 2002, until December 31, 2016. Initially, the screening exposure was defined according to the attendance at the first invitation. The exposure was updated when the screening status of the individual changed during follow-up. A person’s status could move from non-screened to either UGIS- or upper-endoscopy-screened status. In addition, a person who underwent both UGIS and upper endoscopy was included in the upper endoscopy group for analysis, as upper endoscopy is recommended as the final/confirmatory test in the KNCSP.
Outcomes
The cancer diagnosis information was extracted from the KCCR database, which contains all cancer-related characteristics of patients with cancer. The KCCR identified incident forms of cancer cases through the nationwide hospital-based discharge recording system. This hospital-based database covers all general hospitals in Korea that are equipped with a histo-pathology laboratory [13]. The cancer site and tumor location were identified using the International Classification of Disease for Oncology, third edition (ICD-O-3) [17]. Using the topography code, the cancer sites were classified as esophagus cancer (C15.0–C15.9), gastric cancer (C16.0–C16.9), duodenal cancer (C17.0), and other cancers. The term “upper GI cancer” included all esophageal, gastric, or duodenal cancer cases. The tumor behavior was classified as localized, regional, distant, and unknown according to the Surveillance, Epidemiology, and End Results (SEER) summary stage [18].
The date of death and the cause of death were obtained from the Death Certificate from Statistics Korea. The cause of death was identified by the ICD-10 code and was divided into three main subgroups: all-cause death, death from upper GI cancer, and death from other causes except for upper GI cancer.
Statistical analysis
Descriptive statistics were used to describe the population concerning sociodemographic characteristics, including sex, age (5-year age group, mean, standard deviation), socioeconomic status (National Health Insurance Service [NHIS] upper 50% premium, NHIS lower 50% premium, Medical Aid Program). The difference in characteristics between the exposure groups was tested by the chi-square test.
The Cox proportional hazards model, with the exposure of “negative screening result” as a time-varying variable, was used to investigate the association between upper GI cancer incidence and mortality. Whenever the exposure status of individuals changed, their exposure status and age were updated. The hazard ratios (HRs) and the 95% confidence intervals (CIs) were reported to quantify the association in univariate and multivariate models, adjusting for sociodemographic characteristics. For cancer incidence, the person-years were derived from the postal date (January 1, 2002; January 1, 2003) of the first screening invitation to the date of cancer diagnosis or December 31, 2017, for an individual with no cancer record. For analysis of mortality, the person-years were counted until the date of death or December 31, 2019, whichever occurred first. If the screening status changed, the person-year of this patient contributed to all subgroups based on the time that individual stayed in each cohort. The number of individuals was counted twice in both never-screened and screened cohorts but person-years were allocated separately depending on their exposure status (i.e., the date of first attendance for screening).
Additionally, separate models were run for incidence and mortality for each cancer site (esophagus, gastric, and duodenum). Subgroup analyses by sex and age group were performed to examine the features of the association in specific subgroups. Further, to estimate the net effect of the negative screening result on mortality for eliminating the potential selection bias, the HRs for overall mortality and non-upper GI cancer mortality were also reported. Then, the net benefits were calculated using the following formula: net benefit = (HRb-HRa)/HRb \(\times\) 100, where HRb represents the HR for total mortality except upper GI cancer and HRa represents the HR for upper GI cancer mortality. All the statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC), and two-sided P values of < 0.05 denoted statistical significance.
Results
Among 15,850,288 participants, 2,133,547 and 9,392,295 underwent UGIS and upper endoscopy, respectively, through KNCSP and no diagnosis of gastric cancer and other upper GI cancers within 1 year from the date of screening was made (Fig. 1). While there was a larger proportion of males in the control group, approximately 53% of the participants were female and had negative results in the gastric cancer screening group (Table 1). The sociodemographic characteristics were distributed differently according to screening history. By the end of the follow-up time for cancer incidence, 230,783 patients were diagnosed with upper GI cancer in our cohort, which was mostly never-screened men and women (Supplementary Table 1). A total of 209,961 patients had gastric cancer (91%, Supplementary Table 1). A larger proportion of early cancers than late cancers were observed in the screened participants, especially those who underwent endoscopy, for each cancer type (Supplementary Table 1, Supplementary Fig. 1). In detail, 132,440 cases were reported in 123,060,161 person-years given the incidence rate of 10.76 GI cancer cases per 10,000 person-year in the never-screened group. The crude incidence rate was similar in the UGIS group with 10.72 GI cancer cases per 10,000 person-year, whereas the rate was lower in the upper endoscopy group, with 8.32 GI cancer cases per 10,000 person-years (Table 2). The change in the incidence rate over time is shown in Supplementary Fig. 2A. Additionally, a larger proportion of patients with upper GI cancer was detected in the early years of study in the never-screened group. While the largest portion (37%) of patients with GI cancer was detected between the third and fifth year after enrollment in the UGIS group, in most cases, GI cancer occurred after the fifth year of enrollment in the upper endoscopy group (Fig. 2).
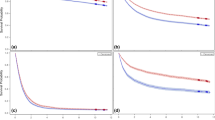
The HRs for upper GI cancer incidence according to screening history are presented in Table 2 and Fig. 3. In the early years of cohort enrollment, the HR of UGIS and upper endoscopy was relatively similar, which then had a wider gap as the HR of the UGIS group moved toward 1 (Fig. 3). By the end of the follow-up period, the people with negative UGIS results had a 19% reduced risk of any upper GI cancer type (aHR = 0.81, 95% CI = 0.80–0.82), and those with negative upper endoscopy results had a 33% reduced risk of the same (aHR = 0.67, 95% CI = 0.67–0.68). In the analysis by cancer site, the risk of esophageal cancer was the lowest, at 23% and 43% for the UGIS and upper endoscopy groups, respectively. Notwithstanding, the risk of other cancers, excluding upper GI cancer, cancers in the KNCSP, lung cancer, and thyroid cancer was relatively similar between screened groups and the never-screened group (UGIS: aHR = 1.04, 95% CI = 1.03–1.05; upper endoscopy: aHR = 0.97, 95% CI = 0.96–0.97) (Table 2).
In the subgroup analysis for cancer incidence, the negative upper endoscopy result group showed the largest risk reduction (Supplementary Table 2). While the effect of negative screening results was similar between men and women, this effect varied among age groups. In the 40–49-year-old age group, the risk of subsequent upper GI cancer was only 5% in the UGIS group (aHR = 0.95, 95% CI = 0.92–0.98) and 12% in the upper endoscopy group (aHR = 0.88, 95% CI = 0.86–0.91), which were the lowest values among all age groups. The effect was the most pronounced for those aged 60–69 years (UGIS: aHR = 0.76, 95% CI = 0.74–0.77; upper endoscopy: aHR = 0.60, 95% CI = 0.59–0.61) (Supplementary Table 2).
In terms of morality, the GI cancer mortality rate was 5.5 per 10,000 person-year (Table 3). Lower crude rates were observed in the UGIS group (4.5 GI cancer death per 10,000 person-year) and upper endoscopy group (1.5 GI cancer death per 10,000 person-year) (Table 3). The mortality rates of each group by year of study are shown in Supplementary Fig. 2B. Regarding HR, while the HR for upper GI cancer mortality increased over time in the UGIS group, the figure was relatively stable over years of study in the upper endoscopy group (Fig. 3B). Overall, the aHRs for upper GI cancer mortality were 0.55 (95% CI = 0.54–0.56) for the negative UGIS result group and 0.21 (95% CI = 0.21–0.22) for the negative upper endoscopy result group (Table 3). The net benefits of upper GI cancer mortality reduction were 21.4% and 54.4% among those with negative UGIS and upper endoscopy results, respectively (Table 3). Additionally, among three types of upper GI cancers, the HRs were the lowest at 0.53 (95% CI = 0.52–0.54) in the UGIS group and 0.19 (95% CI = 0.18–0.20) in the endoscopy group for gastric cancer (Supplementary Table 3). Similar to the results of subgroup analysis by age group for cancer incidence, the 60–69-year-old age group had the lowest risk of upper GI cancer death (Supplementary Table 3).
Discussion
The current study highlighted the significant reduction in the risk of develo** upper GI cancer in persons who received negative screening results, wherein the group with negative upper endoscopy results had a 33% reduced risk of any upper GI cancer. While negative results of both UGIS and upper endoscopy were associated with the decreased risk of mortality from upper GI cancer, a stronger effect was observed in the upper endoscopy group. Very few studies have reported the effect of the negative results of upper GI screening in the average-risk population. A multinational cohort study of a million patients with gastroesophageal reflux disease reported a 55% reduction in the risk of upper GI cancer and a 61% reduction in mortality among people with negative upper endoscopy results [19]. Similarly, Shakhatreh et al. also reported a reduction in the risk of upper GI cancer in patients who received negative upper GI screening results [20]. A Korean study stated that people with negative results on gastric cancer screening had a low risk of cancer. They also found that a never-screened individual had a 1.68-fold higher risk of develo** cancer than an individual who underwent regular endoscopic screening [21]. This effect was reduced in irregularly screened people [21]. Moreover, the mortality reduction due to gastric cancer screening was also well indicated in a previous study of the KNCSP database in South Korea [9,10,11]. Although there was a difference in the study results due to the differences in populations and outcomes, those studies revealed a very similar direction of the effect of screening tests to that observed in our study.
The effect of negative screening in this study could be explained by two aspects. Regarding the risk of cancer, gastric cancer screening, especially endoscopy, is also able to detect various types of precancerous lesions in the GI tract. The removal and treatment of those lesions could prevent them from develo** into the invasive phase; therefore, cancer is prevented [15, 22]. In addition, the screening for Helicobacter pylori (H. pylori), a significant risk factor of gastric cancer [23, 24], is commonly recommended by physicians during visits for upper GI screening. Consequently, detection and successful treatment could reduce the risk of develo** cancer [23]. Furthermore, among screened people, cancer is generally reported in the early stage, with a good prognosis, as documented in previous studies on gastric cancer screening in South Korea [9, 11]. Our results are also in agreement with the results of those studies, wherein early stages of not only gastric cancer (> 80% in the endoscopy group and > 70% in the UGIS group) but also esophageal cancer and duodenal cancer (> 70% in endoscopy group, and > 60% in UGIS group) (Table S1 and Figure S1) were detected most frequently in the group that attended the gastric cancer screening program. These effects resulted in a significant reduction in upper GI mortality in the screened group compared with the never-screened group.
On the other hand, the stronger effect of upper endoscopy than of UGIS is somewhat reasonable as upper endoscopy is currently considered the gold standard for screening and confirmation of any abnormal finding from a radiographic screening test. Regarding performance, an endoscopy allows direct visualization of the GI tract. Accordingly, the KNCSP reported that the UGIS has a lower capability of detecting both cancer and adenomas than the upper endoscopy [25]. Additionally, the limited ability in detecting precancerous lesions suggested the shorter screening interval for the UGIS screening, which was also reflected in our findings. While less than 20% of the cancer cases were detected within 2 years since the receipt of negative screening results from both modalities, 37% of upper GI cancer cases in the negative UGIS group were detected from the third to the fifth year of enrollment. In contrast, in more than 40% of the patients with upper GI cancer who had negative results on endoscopy, the cancer was detected from the sixth to the tenth year (Fig. 2). Thus, for balancing the benefits and harms of screening, the policymakers should also consider adapting the optimal screening interval for each screening modality rather than using the interval of 2 years as set in the current screening guidelines.
Previously, secondary prevention of gastric cancer was considered the main benefit of upper endoscopy and UGIS of the KNCSP. Additional evidence on the effectiveness of gastric cancer screening on esophageal and duodenal cancer may help increase the utility of the KNCSP screening. One of the main barriers to participation in cancer screening programs is the fear of getting cancer [26, 27]. The current evidence on the risk of cancer and death in our study may help relieve this fear in the general population and strengthen their intention to undergo cancer screening and adhere to the recommendations of the KNCSP on the screening interval. Lastly, our findings provide a wide view of the effectiveness of gastric cancer screening, which is already implemented in Asian countries including South Korea and Japan [6,7,8]. While previous studies had addressed the cost-effectiveness of screening by endoscopy or UGIS for gastric cancer alone [14, 28], future studies should also examine the benefit of the whole upper GI tract.
The current study has some limitations, mainly due to its observational nature. Firstly, our study was unable to eliminate selection bias. Patients with upper GI symptoms or a higher risk of GI cancer might prefer to undergo upper endoscopy compared to those without. However, this issue would not explain any associations but instead leads to an underestimation of the protective influence of a negative upper endoscopy and upper GI cancer. To have a better view of this issue, we conducted an additional analysis to estimate the risk of develo** other cancer types excluding upper GI cancer, other cancers in the KNCSP, thyroid cancer, and lung cancer. The relatively similar risk of develo** those cancers indicated that the effect of selection bias on our results would be small. The exclusion of other cancers is explained by the fact that people are generally invited by the KNCSP to screen for serval cancers at a time, and thyroid and lung cancer are the most common opportunistic screening tests in South Korea [29]. Further, in the analysis of upper GI cancer death, the HR for all-cause mortality except upper GI cancer was reported to estimate the net benefit of screening on upper GI cancer mortality reduction. Accordingly, a net benefit of more than 50% was reported for upper endoscopy screening. Second, there was no information on opportunistic screening in our database; hence, the people in the control group may have undergone opportunistic screening tests. Therefore, the effect of the negative screening results may be underestimated and move toward the null. Lastly, as the study covered the target population for gastric cancer screening across the country in 2002–2003, we only included the socioeconomic variables in our model. In particular, H. pylori infection is a well-known factor associated with gastric cancer [24, 30, 31]. The eradication of H. pylori is expected to reduce the occurrence of gastric cancer as indicated by recommendations of previous studies [30,31,32,33]. H. pylori has decreased but the seroprevalence remained relatively high at 51% in Korea [34]. Since KNCSP for stomach cancer is a universal program, we believe there would be no difference in the seroprevalence associated with H. pylori infection between non-participants and participants of KNCSP. Further, a result from a nationwide study in Korea indicated that, in the current context of H. pylori, there is no influence of sociodemographic characteristics as well as the family history of gastric cancer on H. pylori seropositivity, as evidenced by a multivariate analysis [34]. Thus, under the universal cancer screening policy in Korea, the distribution of the infection and eradication of H. pylori may be relatively similar between subgroups. Future studies should consider these factors carefully for better estimation.
Despite the limitations, to the best of our knowledge, our study is the first to use high-quality data from the most reliable and appropriate sources with long follow-up periods to evaluate the effect of the screening intervention at the population level [13]. Therefore, we were able to estimate the real-world effect of the KNCSP screening on the Korean population, which could be generalizable to regions with similar cancer burdens or regions considering the adoption of gastric cancer screening. In conclusion, this population-based study indicates that negative screening, especially on upper endoscopy of the KNCSP, was significantly associated with the reduction in the risk of develo** cancer and death from any type of upper GI cancer.
Data availability
Anonymized data supporting this study’s findings are available from the corresponding author upon request.
References
Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159:335-49.e15.
Arnold M, Pandeya N, Byrnes G, Renehan PAG, Stevens GA, Ezzati PM, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46.
Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31–54.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: cancer today Lyon, France: International Agency for Research on Cancer; 2020. https://gco.iarc.fr/today. Accessed 4 Jul 2022.
Hamashima C. Update version of the Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2018;48:673–83.
Park HA, Nam SY, Lee SK, Kim SG, Shim K-N, Park SM, et al. The Korean guideline for gastric cancer screening. jkma. 2015;58:373–84.
Sugano K. Screening of gastric cancer in Asia. Best Pract Res Clin Gastroenterol. 2015;29:895–905.
Choi KS, Jun JK, Suh M, Park B, Noh DK, Song SH, et al. Effect of endoscopy screening on stage at gastric cancer diagnosis: results of the National Cancer Screening Programme in Korea. Br J Cancer. 2015;112:608–12.
Jun JK, Choi KS, Lee H-Y, Suh M, Park B, Song SH, et al. Effectiveness of the Korean national cancer screening program in reducing gastric cancer mortality. Gastroenterology. 2017;152:1319-28.e7.
Luu XQ, Lee K, Jun JK, Suh M, Jung KW, Choi KS. Effect of gastric cancer screening on long-term survival of gastric cancer patients: results of Korean national cancer screening program. J Gastroenterol. 2022;57:464–75.
Narii N, Sobue T, Zha L, Kitamura T, Iwasaki M, Inoue M, et al. Effectiveness of endoscopic screening for gastric cancer: the Japan public health center-based prospective study. Cancer Sci. 2022. https://doi.org/10.1111/cas.15545.
Hong S, Won Y-J, Lee JJ, Jung K-W, Kong H-J, Im J-S, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021;53:301–15.
Suh YS, Lee J, Woo H, Shin D, Kong SH, Lee HJ, et al. National cancer screening program for gastric cancer in Korea: nationwide treatment benefit and cost. Cancer. 2020;126:1929–39.
Choi KS, Suh M. Screening for gastric cancer: the usefulness of endoscopy. Clin Endosc. 2014;47:490–6.
Ryu JE, Choi E, Lee K, Jun JK, Suh M, Jung KW, et al. Trends in the performance of the Korean national cancer screening program for gastric cancer from 2007 to 2016. Cancer Res Treat. 2021;54:842–9.
World Health Organization. International classification of diseases for oncology (ICD-O)—3rd edition, 1st revision. 3rd ed. Geneva: World Health Organization. 2013.
Young JL Jr, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA. SEER Summary Staging Manual–2000: Codes and Coding Instructions, Bethesda: National Cancer Institute. 2001.
Holmberg D, Santoni G, von Euler-Chelpin MC, Färkkilä M, Kauppila JH, Maret-Ouda J, et al. Incidence and mortality in upper gastrointestinal cancer after negative endoscopy for gastroesophageal reflux disease. Gastroenterology. 2022;162:431-8.e4.
Shakhatreh MH, Duan Z, Avila N, Naik AD, Kramer JR, Hinojosa-Lindsey M, et al. Risk of upper gastrointestinal cancers in patients with gastroesophageal reflux disease after a negative screening endoscopy. Clin Gastroenterol Hepatol. 2015;13:280–6.
Noh CK, Lee E, Lee GH, Lim SG, Park B, Shin SJ, et al. Association of regular endoscopic screening with interval gastric cancer incidence in the national cancer screening program. J Clin Med. 2021;11:230.
Pimentel-Nunes P, Libânio D, Bastiaansen BA, Bhandari P, Bisschops R, Bourke MJ, et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline-Update 2022. Endoscopy. 2022;54(6):591–622.
Kumar S, Metz DC, Ellenberg S, Kaplan DE, Goldberg DS. Risk factors and incidence of gastric cancer after detection of helicobacter pylori infection: a large cohort study. Gastroenterology. 2020;158:527-36.e7.
Polk DB, Peek RM Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–14.
Choi KS, Jun JK, Park EC, Park S, Jung KW, Han MA, et al. Performance of different gastric cancer screening methods in Korea: a population-based study. PLoS ONE. 2012;7: e50041.
Bradley DT, Treanor C, McMullan C, Owen T, Graham A, Anderson D. Reasons for non-participation in the Northern Ireland bowel cancer screening programme: a qualitative study. BMJ Open. 2015;5: e008266.
Hahm M-I, Choi KS, Lee H-Y, Suh M, Lee YY, Shin DW, et al. Do fears of getting cancer and family history of cancer influence participation in opportunistic screening or organized screening for gastric cancer? J Clin Oncol. 2017;35:e13045-e.
Huang HL, Leung CY, Saito E, Katanoda K, Hur C, Kong CY, et al. Effect and cost-effectiveness of national gastric cancer screening in Japan: a microsimulation modeling study. BMC Med. 2020;18:257.
Hong S, Lee YY, Lee J, Kim Y, Choi KS, Jun JK, et al. Trends in cancer screening rates among Korean men and women: results of the Korean national cancer screening survey, 2004–2018. Cancer Res Treat. 2021;53:330–8.
Kim SG, Jung HK, Lee HL, Jang JY, Lee H, Kim CG, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea 2013 revised edition. J Gastroenterol Hepatol. 2014;29:1371–86.
Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, et al. Guidelines for the management of Helicobacter pylori infection in Japan 2009 revised edition. Helicobacter. 2010;15:1–20.
Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–7.
van Grieken NC, Meijer GA, Kale I, Bloemena E, Lindeman J, Offerhaus GJ, et al. Quantitative assessment of gastric antrum atrophy shows restitution to normal histology after Helicobacter pylori eradication. Digestion. 2004;69:27–33.
Lee JH, Choi KD, Jung HY, Baik GH, Park JK, Kim SS, et al. Seroprevalence of Helicobacter pylori in Korea: a multicenter, nationwide study conducted in 2015 and 2016. Helicobacter. 2018;23:e12463.
Acknowledgements
This study was funded by a Grant-in-Aid for Cancer Research and Control from the National Cancer Center, Korea (#2210772).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by Xuan Quy Luu and Kyeongmin Lee. The first draft of the manuscript was written by Xuan Quy Luu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The current study was approved by the Institutional Review Board of the National Cancer Center, Korea (No. NCCNCS08129). Written informed consent was obtained from the participants in the KNCSP database for the collection of their screening results; the requirement for informed consent for the use of the collected data in the current study was waived owing to the use of de-identified data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luu, X.Q., Lee, K., Jun, J.K. et al. Risk of upper gastrointestinal cancer and death in persons with negative screening results: results from the National Cancer Screening Program in South Korea. Gastric Cancer 26, 580–589 (2023). https://doi.org/10.1007/s10120-023-01387-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-023-01387-0