Abstract
Introduction
The accuracy of urine culture results can be affected by pre-analytical factors such as transport delays and storage conditions. The objectives of this study were to analyze urine collection practices and assess the impact of introducing boric acid tubes for urine collection on quantitative urinary bacterial cultures of hospitalized patients in medical wards.
Methods
A quasi-experimental pre-post study conducted in an acute care facility. In the pre-intervention phase (2020–2021), urine samples were transported without preservatives at room temperature. In 2022 (post-intervention), we transitioned to boric acid transport tubes, evaluating its effect on significant bacterial growth (≥ 105 CFU/ml). Bivariate and multivariate analyses identified predictors of culture positivity.
Results
Throughout the duration of the study, a total of 12,660 urine cultures were analyzed. Date and time documentation was complete for 38.3% of specimens. Culture positivity was higher with longer processing times: positivity was 21.3% (220/1034) when specimens were processed within 4 h, 28.4% (955/3364) when processed in 4–24 h, and 32.9% (137/417) when processed after 24 h (p < 0.0001). For 4-24-hour processing, positivity decreased from 30.4% (704/2317) pre-intervention to 24.0% (251/1047) post-intervention (p < 0.001), with no significant changes in < 4 or ≥ 24-hour specimens. Stratified analysis by processing time revealed that the intervention was associated with reduced positivity only in cultures processed within 4–24 h (OR 0.80, 95% CI 0.67–0.94; p = 0.008).
Conclusion
The introduction of boric acid transport tubes predominantly influenced cultures transported within a 4–24-hour window. This presents an opportunity to improve urine tract infection diagnostic practices in healthcare settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary tract infections (UTIs) are among the most prevalent infections in both community and hospital settings. They are linked to substantial morbidity, antibiotic usage, and significant financial expenses [1, 2]. Accurate diagnosis of patients who are exhibiting symptoms of UTI is crucial for applying appropriate antibiotic stewardship, as it helps to prevent both underutilization and overutilization of antimicrobial treatment [3].
A UTI is defined as the growth of bacteria in a urine culture at a concentration above 10 [5] colony forming units per milliliter (CFU/ml) in combination with local or systemic symptoms [4]. Hence, proper collection of urine for culture should have several goals: identify a causative pathogen if present, preserve the organism at a colony count that reflects the patient’s clinical condition at the time of collection, and avoid introduction of a contaminant into the specimen. Overgrowth of a pathogen or mistaking a contaminant for a pathogen may lead to unnecessary antibiotic treatment. The accuracy of urine culture results is susceptible to several pre-analytical factors, such as the method of collection, delay in transport, and storage conditions [5] The gold standard for diagnosis of a UTI is identification of a pathogen in a freshly collected specimen of urine. Previous studies have evaluated the impact of delays in transporting or processing of urine specimens [6, 7] Urine held at room temperature for more than 4 h shows overgrowth of both clinically significant and contaminating microorganisms. This may lead to false-positive results and misdiagnosis of urinary tract infections. Prior research has shown that transport tubes containing boric acid as a preservative effectively inhibit bacterial overgrowth, thus reducing the potential for false-positive results [8,9,10]. However, due to the relatively low number of samples tested, the strength of the evidence was considered low, and the routine use of boric acid tubes for urine sample collection was not recommended [5]. The objectives of this study were to analyze urine collection practices and assess the impact of introducing boric acid tubes for urine collection on quantitative urinary bacterial cultures of hospitalized patients in medical wards.
Methods
The study was conducted at the Wolfson Medical Center, a 720-bed secondary-care teaching hospital situated in central Israel. The facility houses six medical wards with a median patient age of 78 years. Our research was a quasi-experimental pre-post investigation involving all urine cultures collected from patients aged 18 years and older who were hospitalized in medical wards.
During the pre-intervention phase (January 2020-December 2021), Urine cultures were collected in sterile, preservative-free containers, stored at room temperature, and transported to the microbiological laboratory for processing. During the intervention phase (January 2022-December 2022), urine specimens were collected using boric acid tubes (BD Vacutainer® C&S Boric Acid) and transported via the pneumatic transport system.
Laboratory methods
Identical laboratory procedures were applied during both phases. Urine samples were cultured semiquantitatively on Blood Agar / CHROMagar Orientation plates (Hy-Laboratories Ltd, Rehovot, Israel) using the calibrated loop technique and incubated overnight at 37 C°. Each urine culture result was categorized as negative, mixed flora, <100,000 CFU/ml, or ≥ 100,000 CFU/ml (significant bacterial growth). We employed the definition of UTI used for surveillance purposes by the US. National Healthcare Safety Network [11] as well as in several recent studies on. diagnostic stewardship [12,13,14].
Data Collection and definitions
Data on sex, age, presence of indwelling catheter, documentation of date and time of collection, and processing time (i.e., time from specimen collection to the start of incubation) were collected. The processing was categorized as less than 4 h, 4–24 h, or ≥ 24 h. Urine cultures with growth of an organism ≥ 100,000 CFU/mL were considered positive culture results for the purpose of this analysis.
The primary outcome was culture positivity, which is the proportion of cultures with significant bacterial growth.
Statistical analysis
Descriptive statistics were employed to summarize the demographic data and growth rates. Categorical variables were presented as frequencies (%) and continuous variables were represented as median and interquartile range. We used Chi-square to study the associations between categorical variables and having a positive urine culture. Continuous variables were evaluated with either Kruskal-Wallis test or Mann-Whitney test. To identify independent predictors of significant bacterial growth, we used multivariable logistic regression. We repeated the regression, stratifying by processing time. SPSS software was used for all statistical analyses (IBM SPSS Statistics, ver. 28, IBM corp., Armonk, NY, USA, 2021). This study was approved by the Institutional Review Board; the requirement for informed consent was waived.
Results
Patient and urine culture characteristics
During the study period, a total of 12,660 urine cultures were processed, of which 9117 samples were ordered before the intervention and 3543 tests were ordered after the intervention. The characteristics of patients and urine cultures are summarized in Table 1. When comparing the pre-intervention and post-intervention periods, there was a higher proportion of male patients in the post-intervention phase (52.1% vs. 48.5%, p < 0.001). The median age of patients was lower in the post-intervention phase (80 years vs. 81 years, p < 0.001). No significant difference was observed in the prevalence of indwelling urinary catheters.
Complete documentation of collection dates and time was recorded for 38.3% (4830 /12,660) of the specimens. The median processing time has decreased from 14 h (IQR 7–19) to 11 h (IQR 4–7) p < 0.001. During 2022, 26% of urine samples were processed within 4 h, compared to 21% and 16% during 2020 and 2021, respectively (p < 0.001).
Culture positivity
As shown in Table 2, culture positivity was associated with several factors. This included sex, with a positivity rate of 31.0% (763/2458) among females compared with 23.4% (555/2371) among males (p < 0.001); The median age of patients with positive cultures was 83 years (IQR 74–89) compared with 80 years (IQR 69–87) among those with negative or non-significant growth. Samples obtained through indwelling urinary catheters exhibited a positivity rate of 31.2% (962 /3088), while midstream collection yielded a lower rate of 20.9% (332 / 1586). Culture positivity was higher with longer processing times: positivity was 21.3% (220/1034) when specimens were processed within 4 h, 28.4% (955/3364) when processed in 4–24 h, and 32.9% (137/417) when processed after 24 h (p < 0.0001).
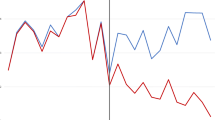
Culture positivity decreased from 28.9% pre-intervention to 23.8% post-intervention (p < 0.001). Among specimens processed within 4–24 h, positivity decreased significantly from 30.4% (704//2317) to 24.0%;(251/1047) while no significant changes were observed for specimens processed < 4 or ≥ 24 h (Fig. 1). A multivariate analysis of fully documented samples revealed multiple variables linked to increase risk of positive results, including age, gender, and presence of indwelling catheter (Table 3). Collection specimens during evening (OR 1.37;95% CI 1.15–1.63; p < 0.001) or night shifts (OR 1.25, 95%CI 1.03–1.52; p = 0.03) were associated with increased positivity compared to day shift. Cultures processed after 24 h were independently associated with increased risk. (OR 1.69, 95%CI 1.30–2.20); p < 0.0001) Further stratified analysis, by processing timing, revealed that the intervention significantly reduced positive culture only for specimens processed between 4 and 24 h (OR 0.80, 95% CI 0.67–0.94; p = 0.008).
Discussion
The objective of this study was to explore urine collection practices and evaluate the impact of utilizing boric acid tubes on quantitative urinary bacterial cultures among hospitalized patients. We found that under standard clinical conditions, the majority of urine cultures were not processed within a 4-hour timeframe. This delay in processing was associated with higher culture positivity. Additionally, increased positivity was observed for cultures collected in the afternoon or night compared to day shifts. The introduction of boric acid transport tubes led to a notable reduction in positivity. Further analysis demonstrated that this intervention had the greatest impact on specimens processed within 4–24 h post-collection.
Delays in specimen transport often challenge laboratories in maintaining the stability of urine samples submitted for culture. Research conducted in the 1970s investigated the impact of transport delays on semiquantitative urine culture results [6, 7]. In line with the findings of these studies, it is recommended to maintain a maximum four-hour interval between urine collection and processing [5]. However, in a survey of US laboratories, only a small number of microbiology laboratories enforced cutoff rule of 4 h for limiting transport time of urine [15]. Notably, in that survey, 90% of the specimens in the surveyed laboratories were received within 9 h. Refrigeration of urine specimens has been shown to limit the overgrowth of organisms [7]. However, temperature-controlled transport is rarely feasible in routine clinical and laboratory settings. Only 35% of laboratories surveyed refrigerated the specimens before or during processing [15]. In the current study, processing time appeared to have a significant impact on culture positivity. We observed an increase in positivity following four hours of sample storage at room temperature, with a more substantial increase noted after 24 h.
Boric acid effectively inhibits the replication of bacteria in urine samples, maintaining the initial quantity of bacteria present at the time of collection. Boric acid preservation has been shown to be comparable to 24 h of refrigeration [16]. Given that the transport duration could exceed the four-hour window, the employment of urine preservatives may be an effective strategy to minimize overgrowth. In one study, boric acid was effective in maintaining the stability of urine specimens for up to 48 h at room temperature [17]. In contrast, Daley et al. found a notable increase in significant bacterial growth when cultures were stored in boric acid tubes for more than 24 h compared to those analyzed immediately. This trend was more evident at 72 h [16]. Likewise, subsequent to the adoption of boric acid transport tubes, our investigation demonstrated a marked reduction in positive cultures only for specimens processed within the 4–24 h window. This suggests a time-dependent effect of boric acid on inhibiting growth, with its potency diminishing after 24 h. Consequently, certain bacterial species may begin to proliferate despite its presence. Therefore, for accurate culture results, urine sample in boric acid tubes should be analyzed within 24 h.
Our investigation has identified notable concerns and opportunities for improvement: A significant portion of collected samples lacked documented collection times. In addition, the majority of specimens were not transported within the recommended 4-hour window. Various factors can contribute to delays in transporting and processing microbiology specimens, potentially jeopardizing patient safety. One such factor is unfamiliarity with the guidelines for timely urine specimen transmission. Additionally, the mode of transportation, whether through manual porters or pneumatic tube systems, can impact the time it takes for samples to reach the laboratory. When pneumatic tube systems are absent, reliance on porter availability becomes a bottleneck, resulting in delays in sample delivery to the lab. Moreover, a significant number of laboratories do not offer 24-hour services. Findings from a survey conducted across four European countries indicated that most laboratories are closed overnight, and merely around 40% maintained services during weekends [18]. Consequently, cultures obtained outside of regular operating hours are often stored for extended periods in clinical wards, typically at room temperature. Our investigation has revealed a clear link between the timing of urine culture collection and the subsequent ratio of positive results. Notably, cultures collected during daytime shifts exhibit lower positivity compared to those acquired during evening and night shifts. These observations indicate that operational hours of the laboratory and the allocation of daily human resources exert a more pronounced influence than the mere growth kinetics of the microorganisms themselves.
The before-after design does not account for other changes in practices that may influence urine culture positivity. During the intervention period, we initiated educational activities aimed at promoting appropriate indications for urine cultures. The effect of this intervention is reflected by the decreased number of cultures collected in 2022, as shown in Table 1. Reducing unnecessary cultures is generally expected to increase urine culture positivity, as noted in a previous study [14]. Therefore, the reduction in positivity observed in the current study is unlikely to be explained by a decrease in urine culture orders.
Our study was subject to various limitations. The research was conducted in a single acute-care hospital, which may limit the generalizability of the findings to other healthcare facilities with different patient populations, practices, and resources. Furthermore, the data collected included minimal information on patient characteristics. The absence of comprehensive patient details, including medical histories and clinical data, potentially overlooks crucial factors that may influence the culture results. The absence of a control group is another potential limitation, making it difficult to ascertain the impact of the laboratory intervention versus other coexisting variables. Nevertheless, it is noteworthy that the reduction in positivity was specifically observed at the 4–24 h processing window, hinting at a plausible connection with the intervention undertaken. Complete documentation was available for only a subset of specimens. Consequently, our ability to assess the impact of processing time was limited to a relatively small portion of the cultures.
In summary, our study underscores the importance for healthcare facilities to re-evaluate and optimize their urine culture transport and storage protocols. We identified substantial delays in transportation, prompting the adoption of boric acid tubes, which resulted in a marked reduction in positivity rates, particularly within the critical 4–24 h processing window. This intervention shows significant potential in enhancing the precision of UTI diagnosis accuracy, thereby contributing to better antibiotic stewardship,
Data availability
No datasets were generated or analysed during the current study.
References
Yang X, Chen H, Zheng Y, Qu S, Wang H, Yi F (2022) Disease burden and long-term trends of urinary tract infections: a worldwide report. Front Public Health 10:888205
Mitchell BG, Ferguson JK, Anderson M, Sear J, Barnett A (2016) Length of stay and mortality associated with healthcare-associated urinary tract infections: a multi-state model. J Hosp Infect 93(1):92–99
Abbo L, Hooton T (2014) Antimicrobial stewardship and urinary tract infections. Antibiotics 3(2):174–192
Wilson ML, Gaido L (2004) Laboratory diagnosis of urinary tract infections in adult patients. CLIN INFECT DIS 38(8):1150–1158
LaRocco MT, Franek J, Leibach EK et al (2016) Effectiveness of Preanalytic practices on Contamination and Diagnostic Accuracy of urine cultures: a Laboratory Medicine Best practices systematic review and Meta-analysis. Clin Microbiol Rev 29(1):105–147
Wheldon DB, Slack M (1977) Multiplication of contaminant bacteria in urine and interpretation of delayed culture. J Clin Pathol 30(7):615
Hindman R, Tronic B, Bartlett R (1976) Effect of delay on culture of urine. J Clin Microbiol 4(1):102–103
Lauer BA, Reller LB, Mirrett S (1979) Evaluation of preservative fluid for urine collected for culture. J Clin Microbiol 10(1):42–45
Lum KT, Meers PD (1989) Boric acid converts urine into an effective bacteriostatic transport medium. J Infect 18(1):51–58
Eriksson I, Lindman R, Thore M (2002) Microbiological evaluation of a commercial transport system for urine samples. Scand J Clin Lab Investig 62(5):325–335
National Healthcare Safety Network website Urinary Tract Infection (Catheter-Associated Urinary Tract Infection [CAUTI] and Non-Catheter-Associated Urinary Tract Infection [UTI]) Events
Luu A, Dominguez F, Yeshoua B et al (2021) Reducing catheter-associated urinary tract infections via cost-saving diagnostic stewardship. Clin Infect Dis 72(11):e883–e886
Yarrington ME, Reynolds SS, Dunkerson T et al (2023) Using clinical decision support to improve urine testing and antibiotic utilization. Infect Control Hosp Epidemiol 44(10):1582–1586
Penney J, Rodday A, Sebastiani P, Snydman D, Doron S (2022) Effecting the culture: impact of changing urinalysis with reflex culture criteria on culture rates and outcomes. ASHE 2(S1):s14–s14
Bekeris LG, Jones BA, Walsh MK, Wagar EA (2008) Urine culture contamination: a College of American pathologists Q-Probes study of 127 Laboratories. Arch Pathol Lab Med 132(6):913–917
Daley P, Gill Y, Midodzi W (2018) Comparison of clinical performance of commercial urine growth stabilization products. Diagn Microbiol Infect Dis 92(3):179–182
Eisinger SW, Schwartz M, Dam L, Riedel S (2013) Evaluation of the BD Vacutainer Plus urine C&S preservative tubes compared with nonpreservative urine samples stored at 4°C and room temperature. Am J Clin Pathol 140(3):306–313
Idelevich EA, Seifert H, Sundqvist M et al (2019) Microbiological diagnostics of bloodstream infections in Europe—an ESGBIES survey. Clin Microbiol Infect 25(11):1399–1407
Acknowledgements
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
Funding
Open access funding provided by Tel Aviv University.
Author information
Authors and Affiliations
Contributions
DB, OS and YC contributed to conception and design of the study. DB wrote the first draft of the manuscript. IZ, OS and YM revised the manuscript. All authors read and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ben-David, D., Cohen, Y., Zohar, I. et al. The impact of Boric Acid tubes on quantitative urinary bacterial cultures in hospitalized patients. Eur J Clin Microbiol Infect Dis (2024). https://doi.org/10.1007/s10096-024-04874-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10096-024-04874-z