Abstract
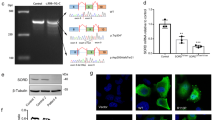
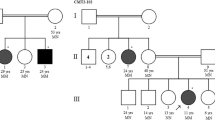
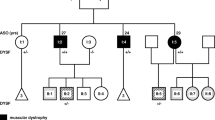
The Dynactin 1 (DCTN1) encodes the p150 subunit of dynactin, which engages retrograde axonal transport. Missense mutations in DCTN1 have been linked to a series of neurodegenerative diseases, including distal hereditary motor neuropathies (dHMN) and Perry syndrome. A few pathogenic DCTN1 mutations related with Perry syndrome have been described within, or adjacent to, the highly conserved N-terminal cytoskeleton-associated protein, glycine-rich (CAP-Gly) domain. But to our best knowledge, only the pathogenic G59S mutation in DCTN1 has been reported in dHMN7B families. Herein, we provided a novel heterozygous mutation in DCTN1 which caused both dHMN7B and Perry syndrome from a Chinese family. Whole exome sequencing (WES) was performed to identify the disease-associated genes. Single nucleotide variants (SNVs) and small insertions/deletions (INDELs) were further predicted with Mutation Taster, Polymorphism Phenoty** v2 (PolyPhen-2), and Sorting Intolerant From Tolerant (SIFT) and compared to the Single Nucleotide Polymorphism Database(dbSNP), Exome Aggregation Consortium (ExAC), and the 1000 Genomes Project. Furthermore, a novel missense mutation c.279G>C (Q93H) in DCTN1 was identified as the candidate loci. The mutation was confirmed with Sanger sequencing in the family members and cosegregated with various phenotypes. In silico analysis and molecular structural modeling, the mutation not only caused the loss of a hydrogen bond within the p150 protein but also affected the formation of hydrogen bonds between p150 and EB. Therefore, the new Q93H mutation in DCTN1 caused both familial dHMN7B and Perry syndrome. Our findings could expand the clinical and pathogenic spectrum and strengthen the clinical diagnostic role of the DCTN1 gene.







Similar content being viewed by others
References
Urnavicius L et al (2015) The structure of the dynactin complex and its interaction with dynein. Science 347(6229):1441–1446
Cronin MA, Schwarz TL (2012) The CAP-Gly of p150: One domain, two diseases, and a function at the end. Neuron 74(2):211–213
Honnappa S et al (2006) Key interaction modes of dynamic +TIP networks. Mol Cell 23(5):663–671
Konno T et al (2017) DCTN1-related neurodegeneration: Perry syndrome and beyond. Parkinsonism Relat Disord 41:14–24
Rossor AM et al (2012) The distal hereditary motor neuropathies. J Neurol Neurosurg Psychiatry 83(1):6–14
Puls I et al (2003) Mutant dynactin in motor neuron disease. Nat Genet 33(4):455–456
Hwang SH et al (2016) Distal hereditary motor neuropathy type 7B with Dynactin 1 mutation. Mol Med Rep 14(4):3362–3368
Nam SH et al (2016) Identification of genetic causes of inherited peripheral neuropathies by targeted gene panel sequencing. Mol Cell 39(5):382–388
Tian WT et al (2020) New phenotype of DCTN1-related spectrum: early-onset dHMN plus congenital foot deformity. Ann Clin Transl Neurol 7(2):200–209
Chae JH et al (2015) Utility of next generation sequencing in genetic diagnosis of early onset neuromuscular disorders. J Med Genet 52(3):208–216
Schabhüttl M et al (2014) Whole-exome sequencing in patients with inherited neuropathies: outcome and challenges. J Neurol 261(5):970–982
Klein CJ et al (2014) Application of whole exome sequencing in undiagnosed inherited polyneuropathies. Journal of Neurology. Neurosurg Psychiatry 85(11):1265–1272
Farrer MJ et al (2009) DCTN1 mutations in Perry syndrome. Nat Genet 41(2):163–165
Wider C et al (2010) Elucidating the genetics and pathology of Perry syndrome. J Neurol Sci 289(1–2):149–154
Tsuboi Y et al (2008) Neurodegeneration involving putative respiratory neurons in Perry syndrome. Acta Neuropathol 115(2):263–268
Wider C et al (2009) Pallidonigral TDP-43 pathology in Perry syndrome. Parkinsonism Relat Disord 15(4):281–286
Richards S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424
Schroer TA (2004) Dynactin. Annu Rev Cell Dev Biol 20(1):759–779
Waterman-Storer CM, Karki S, Holzbaur EL (1995) The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1). Proc Natl Acad Sci U S A 92(5):1634–1638
Li Y, Garrett G, Zealear D (2017) Current treatment options for bilateral vocal fold paralysis: a state-of-the-art review. Clin Exp Otorhinolaryngol 10(3):203–212
Araki E et al (2014) A novel DCTN1 mutation with late-onset parkinsonism and frontotemporal atrophy. Mov Disord 29(9):1201–1204
Tacik P et al (2014) Three families with Perry syndrome from distinct parts of the world. Parkinsonism Relat Disord 20(8):884–888
Dixit R et al (2008) Regulation of dynactin through the differential expression of p150GluedIsoforms. J Biol Chem 283(48):33611–33619
Stockmann M et al (2013) The dynactin p150 subunit: cell biology studies of sequence changes found in ALS/MND and Parkinsonian syndromes. J Neural Transm (Vienna) 120(5):785–798
Moughamian AJ, Holzbaur ELF (2012) Dynactin is required for transport initiation from the distal axon. Neuron 74(2):331–343
Perry TL et al (1990) Dominantly inherited apathy, central hypoventilation, and Parkinson’s syndrome: clinical, biochemical, and neuropathologic studies of 2 new cases. Neurology 40(12):1882–1887
Cooper-Knock J et al (2017) Targeted genetic screen in amyotrophic lateral sclerosis reveals novel genetic variants with synergistic effect on clinical phenotype. Front Mol Neurosci 10:370
Kuźma-Kozakiewicz M et al (2013) Dynactin deficiency in the CNS of humans with sporadic ALS and mice with genetically determined motor neuron degeneration. Neurochem Res 38:2463–2473
Tanaka F et al (2012) Neuropathology and omics in motor neuron diseases. Neuropathology 32(4):458–462
Bercier V et al (2019) Dynactin1 depletion leads to neuromuscular synapse instability and functional abnormalities. Mol Neurodegener 14(1):27
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical publication statement
We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this study is consistent with those guidelines.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were approved by the Ethics committee of First Hospital of Shanxi Medical University (Taiyuan, China) and carried out in accordance with the principles of Helsinki Declaration.
Informed consent
Informed consent was preliminarily obtained from all patients and controls before their inclusion in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 2
(XLS 29 kb)
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, H., Liu, W. et al. A novel Q93H missense mutation in DCTN1 caused distal hereditary motor neuropathy type 7B and Perry syndrome from a Chinese family. Neurol Sci 42, 3695–3705 (2021). https://doi.org/10.1007/s10072-020-04962-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04962-w