Abstract
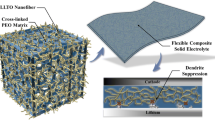
Due to superior energy density and safety, all-solid-state lithium-ion batteries (ASSLBs) are considered to be the perfect substitute for lithium-ion batteries. As the most important component of ASSLBs, solid-state electrolytes (SSEs) are an important part of promoting the commercial development of ASSLBs. However, low ionic conductivity and poor oxidation stability of SSEs are the main obstacles to their industrial preparation. Here, we chose garnet Li7La3Zr2O12 ceramics to prepare a three-dimensional (3D) rebar-like structure nanofiber framework by electrospinning, and infiltrating a polyethylene oxide (PEO) polymer matrix to form the reinforced concrete-like composite solid electrolytes (3D RC-CSEs). The regularly arranged and interconnected framework endows the 3D RC-CSEs with a fast ion transport channel (ion conductivity is 0.23 mS cm−1 at 30 ℃), the lithium-ion transference number is up to 0.47 and electrochemical stability window of 4.9 V (vs. Li+/Li). The Li|3D RC-CSEs|Li battery can achieve a stable cycle for 3200 h at 0.2 mA cm−2. The LFP|3D RC-CSEs|Li battery shows an excellent rate performance (discharge specific capacity of 123.1 mAh g−1 at 3 C) and great cycling performance (discharge specific capacity of 123.1 mAh g−1 after 600 cycles at 1 C) at 60 ℃. In addition, the 3D RC-CSEs showed that Young's modulus is 9.6 times of the PEO-LiTFSI. The unique structural design provides a practical strategy for commercial development.







Similar content being viewed by others
References
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Wang Q, ** P, Zhao X, Chu G, Sun J, Chen C (2012) Thermal runaway caused fire and explosion of lithium ion battery. J Power Sources 208:210–224
Schnell J, Günther T, Knoche T, Vieider C, Köhler L, Just A, Keller M, Passerini S, Reinhart G (2018) All-solid-state lithium-ion and lithium metal batteries-paving the way to large-scale production. J Power Sources 382:160–175
Manthiram A, Yu X, Wang S (2017) Lithium battery chemistries enabled by solid-state electrolytes. Nat Rev Mater 2:1–16
Bachman JC, Muy S, Grimaud A, Chang HH, Pour N, Lux SF, Paschos O, Maglia F, Lupart S, Lamp P, Giordano L (2016) Shao-Horn, Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem Rev 116:140–162
Goodenough JB, Singh P (2015) Review-solid electrolytes in rechargeable electrochemical cells. J Electrochem Soc 162:A2387–A2392
Cheng XB, Zhang R, Zhao CZ, Zhang Q (2017) Toward safe lithium metal anode in rechargeable batteries: a review. Chem Rev 117:10403–10473
Guo Y, Li H, Zhai T (2017) Reviving lithium-metal anodes for next-generation high-energy batteries. Adv Mater 29:1–25
Liu Y, Xu B, Zhang W, Li L, Lin Y, Nan C (2020) Composition modulation and structure design of inorganic-in-polymer composite solid electrolytes for advanced lithium batteries. Small 16:1902813
Agrawal RC, Pandey GP (2008) Solid polymer electrolytes: materials designing and all-solid-state battery applications: an overview. J Phys D 41:3715–3725
Saxena AG, Kamaraj SK, Caballero-Briones F (1998) Polymer electrolytes for lithium ion batteries. Adv Mater 10(6):439–448
Liu LH, Lyu J, Mo JS, Peng P, Li JG, Jiang B, Chu LH, Li MC (2020) Flexible, high-voltage, ion-conducting composite membranes with 3D aramid nanofiber frameworks for stable all-solid-state lithium metal batteries. Sci China Mater 65:703–718
Khurana R, Schaefer JL, Archer LA, Coates GW (2014) Suppression of lithium dendrite growth using cross-linked polyethylene/poly(ethylene oxide) electrolytes: a new approach for practical lithium-metal polymer batteries. J Am Chem Soc 136:7395–7402
Zheng Q, Ma L, Khurana R, Archer LA, Coates GW (2016) Structure-property study of cross-linked hydrocarbon/poly(ethylene oxide) electrolytes with superior conductivity and dendrite resistance. Chem Sci 7:6832–6838
Niitani T, Shimada M, Kawamura K, Dokko K, Rho YH, Kanamura K (2005) Synthesis of Li+ Ion conductive PEO-PSt block copolymer electrolyte with microphase separation structure. Electrochem Solid-State Lett 8:A385–A388
Donald R, Sadoway Huang B, Patrick E (2001) Self-doped block copolymer electrolytes for solid-state, rechargeable lithium batteries. J Power Sources 97(8):621–623
Devaux D, Glé D, Phan TNT, gigmes D, Giroud E, Deschamps M, Denoyel R, Bouchet R (2015) Optimization of block copolymer electrolytes for lithium metal batteries. Chem Mater 27:4682–4692
Cheruvally G, Kim JK, Choi JW, Ahn JH, Shin YJ, Manuel J, Raghavan P, Kim KW, Ahn HJ, Choi DS (2007) Electrospun Polymer membrane activated with room temperature ionic liquid: novel polymer electrolytes for lithium batteries. J Power Sources 172:863–869
Zhang M, Zhang J, Yang J, Du X, Cui G (2019) Polymer electrolyte enlightens wide-temperature 4.45V-class LiCoO2/Li metal battery. J Electrochem Soc 166:A2313–A2321
Feuillade G, Perche P (1975) Ion-conductive macromolecular gels and membranes for solid lithium cells. J Appl Electrochem 5:63–69
Liu YT, Tao XY, Wang Y, Jiang C, Ma C, Sheng O, Lu GX, Lou XW (2022) Self-assembled monolayers direct a LiF-rich interphase toward long-life lithium metal batteries. Science 375:739–745
Hong JC, Dong WK, Park JW, Park JH, Lee YJ, Ha YC, Lee SM, Yoon SY, Kim BG (2022) In situ formed Ag-Li Intermetallic layer for stable cycling of all-solid-state lithium batteries. Adv Sci 9:2270001
Munakata H, Wakasugi, Jungo, Kanamura, Kiyoshi (2017) Effect of gold layer on interface resistance between lithium metal anode and Li6.25Al0.25La3Zr2O12 solid electrolyte. J Electrochem Soc 164:A1022–A1025
Yang CP, **e H, ** WW, F K, Liu BY, Rao JC, Dai JQ, Wang CW, Pastel G, Hu LB (2019) An electron/ion dual-conductive alloy framework for high-rate and high-capacity solid-state lithium-metal batteries. Adv Mater 31:1–7
Lu Y, Huang X, Yadong R, Wang Q, Rui K, Yang J, Wen Z (2018) An in situ element permeation constructed high endurance Li-LLZO interface at high current densities. J Mater Chem A 6:18853–18858
Zhao N, Fang R, He MH, Chen C, Li YQ, Bi ZJ, Guo XX (2018) Cycle stability of lithium/garnet/lithium cells with different intermediate layers. Rare Met 37:473–479
Cheng Z, Liu T, Zhao B, Shen F, ** H, Han X (2021) Recent advances in organic-inorganic composite solid electrolytes for all-solid-state lithium batteries. Energy Storage Mater 34:388–416
Cai M, ** J, **u T, Song Z, Badding ME, Wen Z (2022) In-situ constructed lithium-salt lipophilic layer inducing bi-functional interphase for stable LLZO/Li interface. Energy Storage Mater 47:61–69
Bae J, Li Y, Zhao F, Zhou X, Ding Y, Yu G (2018) Designing 3D nanostructured garnet frameworks for enhancing ionic conductivity and flexibility in composite polymer electrolytes for lithium batteries. Energy Storage Mater 15:46–52
Wu MJ, Liu D, Qu DY, **e ZZ, Li JS, Lei JH, Tang HL (2020) 3D coral-like LLZO/PVDF composite electrolytes with enhanced ionic conductivity and mechanical flexibility for solid-state lithium batteries. Acs Appl Mater Inter 12:52652–52659
Fu KK, Gong Y, Dai J, Gong A, Han X, Yao Y, Wang C, Wang Y, Chen Y, Yan C, Li Y, Wachsman ED, Hu L (2016) Flexible, solid-state, ion-conducting membrane with 3D garnet nanofiber networks for lithium batteries. Proc Natl Acad Sci USA 113:7094–7099
**e H, Yang C, Ren Y, Xu S, Hamann TR, McOwen DW, Wachsman ED, Hu L (2021) Amorphous-carbon-coated 3D solid electrolyte for an electro-chemomechanically stable lithium metal anode in solid-state batteries. Nano Lett 21:6163–6170
Gao L, Luo S, Li J, Cheng B, Kang W, Deng NJESM (2021) Core-shell structure nanofibers-ceramic nanowires based composite electrolytes with high Li transference number for high-performance all-solid-state lithium metal batteries. Energy Storage Mater 43:266–274
Chen L, Li Y, Li S-P, Fan L-Z, Nan C-W, Goodenough JB (2018) PEO/garnet composite electrolytes for solid-state lithium batteries: from ceramic-in-polymer to polymer-in-ceramic. Nano Energy 46:176–184
Gao LW, Deng N (2022) Optimized CeO2 nanowires with rich surface oxygen vacancies enable fast Li-ion conduction in composite polymer electrolytes. Energy & Environmental Materials 6:e12272
Pan P, Zhang M, Cheng Z, Jiang L, Mao J, Ni C, Chen Q, Zeng Y, Hu Y, Fu K (2022) Garnet ceramic fabric-reinforced flexible composite solid electrolyte derived from silk template for safe and long-term stable all-solid-state lithium metal batteries. Energy Storage Mater 47:279–287
Rangasamy E, Wolfenstine J, Sakamoto J (2012) The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid State Ion 206:28–32
Wang X, Huang S, Guo K, Min Y, Xu Q (2022) Directed and continuous interfacial channels for optimized ion transport in solid-state electrolytes. Adv Funct Mater 32:2206976
Liu M, Zhang SG, van Eck ERH, Wang C, Ganapathy S, Wagemaker M (2022) Improving Li-ion interfacial transport in hybrid solid electrolytes. Nat Nanotechnol 17:959–967
Zagórski J, López del Amo JM, Cordill MJ, Aguesse F, Buannic L, Llordés A (2019) Garnet-Polymer Composite electrolytes: New insights on Local Li-Ion dynamics and electrodeposition stability with li metal anodes. Acs Appl Energ Mater 2:1734–1746
Ding Y, Shen X, Zeng J, Wang X, Peng L, Zhang P, Zhao J (2018) Pre-irradiation grafted single lithium-ion conducting polymer electrolyte based on poly(vinylidene fluoride). Solid State Ion 323:16–24
Li J, Deepak FL (2022) Situ kinetic observations on Crystal Nucleation and Growth. Chem Rev 122:16911–16982
Liang H, Wang L, Wang A, Song Y, Wu Y, Yang Y, He X (2023) Tailoring practically accessible polymer/inorganic composite electrolytes for all-solid-state lithium metal batteries: A review. Nanomicro Lett 15:42
Ma Y, Wan J, Yang Y, Ye Y, **ao X, Boyle DT, Burke W, Huang Z, Chen H, Cui Y, Yu Z, Oyakhire ST, Cui Y (2022) Scalable, ultrathin, and high-temperature‐resistant solid polymer electrolytes for energy‐dense lithium metal batteries. Adv Energy Mater 12:2103720
Funding
This work was supported by the Natural Science Foundation of Tian** (No. 20JCYBJC00230). The authors are thankful to the Analytical & Testing Center of Tiangong University for the technical support in XRD, SEM, and XPS measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruan, Y., Feng, J., Huang, X. et al. A novel reinforced concrete-like composite solid-state electrolyte with enhanced performance for all-solid-state lithium batteries. J Solid State Electrochem (2024). https://doi.org/10.1007/s10008-024-05820-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10008-024-05820-x