Abstract
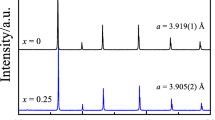
High-temperature electrical conductivity and electrochemical activity in the oxygen redox reaction of Sr2FeCo0.5Mo0.5O6-δ (SFCM) and Sr1.6La0.4FeCo0.5Mo0.5O6-δ (LSFCM) at variable oxygen partial pressure have been studied. We have found that the partial replacement of Sr2+ by La3+ results in a substantial decrease in the total electrical conductivity due to a decrease in the hole charge carrier concentration. Detailed analysis of the high- and low-frequency parts of the impedance spectra at pO2 = 0.1–1 atm and 873–1173 K has revealed different rate-limiting steps in the oxygen redox reaction for SFCM and LSFCM resulted from different oxygen vacancy concentrations in these materials. For SFCM, the oxygen redox reaction is limited by the processes of adsorption and dissociation of oxygen molecules, while for LSFCM by the charge transfer occurring at the triple phase boundary.








Similar content being viewed by others
References
Ruiz-Morales JC, Marrero-Lopez D, Canales-Vazquez J, Irvine JTA (2011) Symmetric and reversible solid oxide fuel cells. RSC Adv 1:1403–1414. https://doi.org/10.1039/C1RA00284H
Su C, Wang W, Liu M, Tadé MO, Shao Z (2015) Progress and prospects in symmetrical solid oxide fuel cells with two identical electrodes. Adv Energy Mater 1500188. https://doi.org/10.1002/aenm.201500188
Istomin SY, Lyskov NV, Mazo GN, Antipov EV (2021) Electrode materials based on complex d-metal oxides for symmetrical solid oxide fuel cells. Russ Chem Rev 90:644–676. https://doi.org/10.1070/RCR4979
Skutina L, Filonova E, Medvedev D, Maignan A (2021) Undoped Sr2MMoO6 double perovskite Molybdates (M = Ni, Mg, Fe) as promising anode materials for solid oxide fuel cells. Materials 14:1715. https://doi.org/10.3390/ma14071715
Liu Q, Dong X, **ao G, Zhao F, Chen F (2010) A novel electrode material for symmetrical SOFCs. Adv Mater 22:5478. https://doi.org/10.1002/adma.201001044
**ao G, Liu Q, Zhao F, Zhang L, **a C, Chen F (2011) Sr2Fe1.5Mo0.5O6 as cathodes for intermediate-temperature solid oxide fuel cells with La0.8Sr0.2Ga0.87Mg0.13O3 electrolyte. J Electrochem Soc 158:B455. https://doi.org/10.1149/1.3556085
Li H, Tian Y, Wang Z, Qie F, Li Y (2021) An all perovskite direct methanol solid oxide fuel cell with high resistance to carbon formation at the anode. RSC Adv 2:3857–3863. https://doi.org/10.1039/C2RA01256A
Goodenough JB, Huang YH (2007) Alternative anode materials for solid oxide fuel cells. J Power Sources 173:1–10. https://doi.org/10.1016/j.jpowsour.2007.08.011
Wei T, Zhang Q, Huang YH, Goodenough JB (2012) Cobalt-based double-perovskite symmetrical electrodes with low thermal expansion for solid oxide fuel cells. J Mater Chem 22:225–231. https://doi.org/10.1039/C1JM14756K
Pan X, Wang Z, He B, Wang S, Wu X, ** on the electrochemical properties of Sr2Fe1.5Mo0.5O6 electrode for solid oxide fuel cell. Int J Hydrogen Energy 38:4108–4115. https://doi.org/10.1016/j.ijhydene.2013.01.121
Zhen S, Sun W, Tang G, Rooney D, Sun K, Ma X (2016) Evaluation of strontium-site-deficient Sr2Fe1.4Co0.1Mo0.5O6−δ-based perovskite oxides as intermediate temperature solid oxide fuel cell cathodes. Int J Hydrogen Energy 41:9538–9546. https://doi.org/10.1016/j.ijhydene.2016.04.094
Song Y, Zhong Q, Tan W, Pan C (2014) Effect of cobalt-substitution Sr2Fe1.5-xCoxMo0.5O6−δ for intermediate temperature symmetrical solid oxide fuel cells fed with H2–H2S. Electrochim Acta 139:13–20. https://doi.org/10.1016/j.electacta.2014.07.022
Song Y, Zhong Q, Wang D, Xu Y, Tan W (2017) Interaction between electrode materials Sr2FeCo0.5Mo0.5O6−δ and hydrogen sulfide in symmetrical solid oxide fuel cells. Int J Hydrogen Energy 42:22266–22272. https://doi.org/10.1016/j.ijhydene.2017.04.216
Yang Y, Wang Y, Yang Zh, Lei Z, ** Ch, Liu Y, Wang Y, Peng S (2019) Co-substituted Sr2Fe1.5Mo0.5O6-δ as anode materials for solid oxide fuel cells: achieving high performance via nanoparticle exsolution. J Power Sources 438:226989. https://doi.org/10.1016/j.jpowsour.2019.226989
Abdullaev MM, Istomin SY, Sobolev AV, Presnyakov IA, Antipov EV (2019) Synthesis and study of (Sr, La)2FeCo0.5Mo0.5O6−δ oxides with double perovskite structure. Russ J Inorg Chem 64:696–704. https://doi.org/10.1134/S0036023619060032
Merkulov OV, Markov AA, Patrakeev MV, Leonidov IA, Shalaeva EV, Tyutyunnik AP, Kozhevnikov VL (2018) Structural features and high-temperature transport in SrFe0.7Mo0.3O3−δ. J Solid State Chem 258:447–452. https://doi.org/10.1016/j.jssc.2017.11.008
Hong DJL, Smyth DM (1992) Defect chemistry of undoped La2CuO4. J Solid State Chem 97:427–433. https://doi.org/10.1016/0022-4596(92)90052-W
Patrakeev MV, Leonidov IA, Kozhevnikov VL, Kharton VV (2004) Ion–electron transport in strontium ferrites: relationships with structural features and stability. Solid State Sci 6:907–913. https://doi.org/10.1016/j.solidstatesciences.2004.05.002
Barsoukov E, Macdonald JR (2005) Impedance spectroscopy: theory, experiment, and applications. John Wiley & Sons, New Jersey. https://doi.org/10.1002/0471716243
Takeda Y, Kanno R, Noda M, Tomida Y, Yamamoto O (1987) Cathodic polarization phenomena of perovskite oxide electrodes with stabilized zirconia. J Electrochem Soc 134:2656. https://doi.org/10.1149/1.2100267
Kenney B, Karan K (2006) Impact of nonuniform potential in SOFC composite cathodes on the determination of electrochemical kinetic parameters: a numerical analysis. J Electrochem Soc 153:A1172. https://doi.org/10.1149/1.2191187
Chen XJ, Khor KA, Chan SH (2003) Identification of O2 reduction processes at yttria stabilized zirconia|doped lanthanum manganite interface. J Power Sources 123:17–25. https://doi.org/10.1016/S0378-7753(03)00436-1
Jiang Z, Lei Z, Ding B, **a C, Zhao F, Chen F (2010) Electrochemical characteristics of solid oxide fuel cell cathodes prepared by infiltrating (La, Sr)MnO3 nanoparticles into yttria-stabilized bismuth oxide backbones. Int J Hydrogen Energy 35:8322–8330. https://doi.org/10.1016/j.ijhydene.2009.12.008
Adler SB (1998) Mechanism and kinetics of oxygen reduction on porous La1−xSrxCoO3−δ electrodes. Solid State Ionics 111:125–134. https://doi.org/10.1016/S0167-2738(98)00179-9
Osinkin DA, Khodimchuk AV, Porotnikova NM, Bogdanovich NM, Fetisov AV, Ananyev MV (2020) Rate-determining steps of oxygen surface exchange kinetics on Sr2Fe1.5Mo0.5O6−δ. Energies 13:250. https://doi.org/10.3390/en13010250
Funding
This work has been financially supported by RFBR project # 20–03-00432 and partially by the Interdisciplinary Scientific and Educational School of Moscow University “Future Planet: Global Environmental Changes.” N.V.L. is grateful for financial support the state task for IPCP RAS, state registration no. AAAA-A19-119061890019–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdullaev, M.M., Lyskov, N.V., Istomin, S.Y. et al. High-temperature electrical conductivity and electrochemical activity in oxygen redox reaction of La-doped Sr2FeCo0.5Mo0.5O6-δ. J Solid State Electrochem 26, 2771–2779 (2022). https://doi.org/10.1007/s10008-022-05284-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-022-05284-x