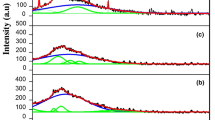
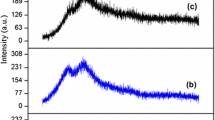
Abstract
Solid polymer electrolyte based on iota-carrageenan (i-carrageeenan) with ammonium thiocyanate (NH4SCN) has been prepared by solution casting technique using distilled water as solvent. Increase of amorphous nature of the polymer/salt complex has been confirmed by XRD analysis. The complex formation between the polymer and salt has been confirmed by FTIR analysis. A shift in glass transition temperature (Tg) of the i-carrageeenan/ NH4SCN electrolytes has been observed from the DSC thermograms. From AC impedance spectroscopy, the maximum conductivity value has been found to be 3.56 × 10−3 S/cm for i-carrageeenan (1 g): NH4SCN (0.3 wt%) at room temperature. Also it has been observed that the activation energy evaluated from the Arrhenius plots has been found to be low (0.21 eV) for i-carrageeenan (1 g): NH4SCN (0.3 wt%) polymer electrolyte. The ionic transference number has been measured using DC Wagner’s polarization method for highest conducting polymer membrane and the result indicates that the conductivity of the electrolyte is predominantly due to ions. The electrochemical stability of the electrolyte i-carrageeenan (1 g): NH4SCN (0.3 wt%) has been studied by linear sweep voltammetry. Using this maximum ionic conductivity polymer electrolyte, the proton battery and fuel cell have been constructed and the cell parameters have been reported.

Possible interaction between i-carrageeenan and NH4SCN.













Similar content being viewed by others
References
Johansson B (2013) A broadened typology on energy and security. Energy 53:199–205
Monisha S, Mathavan T, Selvasekarapandian S, Milton Franklin Benial A, Premalatha M (2016) Preparation and characterization of cellulose acetate and lithium nitrate for advanced electrochemical devices. Ionics 23(10):2697–2706
Harun NI, Ali RM, Ali AMM, Yahya MZA (2012) Dielectric behaviour of cellulose acetate-based polymer electrolytes. Ionics 18(6):599–606
Shukur MF, Kadir MFZ (2015) Electrical & transport properties of NH4Br doped corn starch based solid biopolymer electrolyte. Ionics 21(1):111–124
Tiwari T, Srivastava N, Srivastava PC (2013) Ion dynamics study of potato starch β sodium salts electrolyte system. International Journal of Electrochemistry 2013:8
Vijaya N, Selvasekarapandian S, Sornalatha S, Sujithra KS, Monisha S (2016) Proton-conducting biopolymer electrolytes based on pectin doped with NH4X (X=Cl, Br). Ionics 10:2799–2808
Leones R, Botelho MBS, Sentanin F, Cesarino I, Pawlicka A, Camargo ASS, Silva MM (2014) Pectin-based polymer electrolytes with Ir (III) complexes. Mol Cryst Liq Cryst 604(1):117–125
Samsudin AS, Isa MIN (2012) Structural and ionic transport study on CMC doped NH4Br: a new types of biopolymer electrolytes. J Appl Sci 12:174–179
Ramlli MA, Isa MIN (2014) Conductivity study of carboxyl methyl cellulose solid biopolymer electrolytes (SBE) doped with ammonium fluoride. Res J Recent Sci 3(6):59–66
Majid SR, Arof AK (2005) Proton-conducting polymer electrolyte films based on chitosan acetate complexed with NH4NO3 salt. Phys B Condens Matter 355(1–4):78–82
Nawaz A, Sharif R, Rhee HW, Singh PK (2016) Efficient dysensitized solar cell and supercapacitor using1-ethyl 3-methylimidazoliumdicyanamidein- corporate PVDF-HFP polymer matrix. J Ind Eng Chem 33:381–384
Selvalakshmi S, Vijaya N, Selvasekarapandian S, Premalatha M (2017) Biopolymer agar-agar doped with NH4SCN as solid polymer electrolyte for electrochemical cell application. J Appl Polym Sci 134:44702
Siti SA, Ahmad AM (2013) Effect of NH4I and I2 concentration on agar gel polymer electrolyte properties for a dye-sensitized solar cell. Ionics 19:1185–1194
Masao K, Takayuki H, Yuuki K, Hirohito U (2004) Solid type dye-sensitized solar cell using polysaccharide containing redox electrolyte solution. J Electroanal Chem 572:21–27
Shuhaimi NEA, Alias NA, Majid SR, Arof AK (2008) Electrical double layer capacitor with proton conducting Κ-Carrageenan–Chitosan electrolytes. Funct Mater Lett 01(03):195–201
Rudhziah S, Rani MSA, Ahmad A, Mohamed NS, Kaddami H (2015) Potential of blend of kappa-carrageenan and cellulose derivatives for green polymer electrolyte application. Ind Crop Prod 72:133–141
Karthikeyan S, Selvasekarapandian S, Premalatha M, Monisha S, Boopathi G, Aristatil G, Arun A, Madeswaran S (2016) Proton-conducting i-carrageeenan-based biopolymer electrolyte for fuel cell application. Ionics 10:2775–2780
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Boopathi G (2018) Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J Non-Cryst Solids 481:424–434
Prasad K, Kaneko Y, Kadokawa J (2009) Novel gelling systems of kappa-, iota- and lambda-carrageenans and their composite gels with cellulose using ionic liquid. Macromol Biosci 9(4):376–382
Sridevi D, Rajendran KV (2009) Synthesis and optical characteristics of ZnO nanocrystals. Bull Master Sci 32(2):165–168
Kumar V, Sonkawade RG, Dhaliwal AS (2012) High electronic excitation induced modifications by 100MeV O7+ and 150MeV Ni11+ ions in Makrofol KG polycarbonate film. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 287:4–9
Vij A, Chawla AK, Kumar R, Lochab SP, Chandra R, Singh N (2010) Effect of 120 MeV Ag9+ ion beam irradiation on the structure and photoluminescence of SrS:Ce nanostructures. Phys B 405(11):2573–2576
Pereira L, Amado AM, Critchley AT, Van De Velde F, Ribeiro-Claro PJA (2009) Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocoll 23(7):1903–1909
Rajeswari N, Selvasekarapandian S, Karthikeyan S, Sanjeeviraja C, Iwai Y, Kawamura J (2013) Structural, vibrational, thermal, and electrical properties of PVA/PVP biodegradable polymer blend electrolyte with CH3COONH4. Ionics 19(8):1105–1113
Brychcy E, Malik M, Drożdżewski P, Krol Z, Jarmoluk A (2015) Physicochemical and antibacterial properties of carrageenan and gelatine hydrosols and hydrogels incorporated with acidic electrolyzed water. Polymers 7(12):2638–2649
Maurya KK, Srivastava N, Hashmi SA, Chandra S (1992) Proton conducting polymer electrolyte: II poly ethylene oxide + NH4l system. J Mater Sci 27(23):6357–6364
Rajendran S, Sivakumar M, Subadevi R (2004) Li-ion conduction of plasticized PVA solid polymer electrolytes complexed with various lithium salts. Solid State Ionics 167(3-4):335–339
Martins JT, Cerqueira MA, Bourbon AI, Pinheiro AC, Souza BWS, Vicente AA (2012) Synergistic effects between κ-carrageenan and locust bean gum on physicochemical properties of edible films made thereof. Food Hydrocoll 29(2):280–289
Campo VL, Kawano DF, da Silva DB Jr, Carvalho I (2009) Carrageenans: biological properties, chemical modifications and structural analysis—a review. Carbohydr Polym 77(2):167–180
Hema M, Selvasekarapandian S, Hirankumar G, Sakunthala A, Arunkumar D, Nithya H (2010) Laser Raman and ac impedance spectroscopic studies of PVA: NH4NO3 polymer electrolyte. Spectrochim Acta A 75(1):474–478
Vargas RA, Garcia A, Vargas MA (1998) Phase behaviour of complexes of PVA and acid salts. Electrochim Acta 43(10-11):1271–1274
Monisha S, Selvasekarapandian S, Mathavan T, Milton Franklin Benia A, Sindhuja M, Karthikeyan S (2016) Preparation and characterization of biopolymer electrolyte based on cellulose acetate for potential applications in energy storage devices. J Mater Sci Mater Electron 27(9):9314–9324
Premalatha M, Mathavan T, Selvasekarapandian S, Monisha S, Vinoth Pandi D, Selvalakshmi S (2016) Investigations on proton conducting biopolymer membranes based on tamarind seed polysaccharide incorporated with ammonium thiocyanate. J Non-Cryst Solids 453:131–140
Mertens IJA, Wubbenhorst M, Oosterbaan WD, Jenneskens LW, Turnhout JV (1999) Novel polymer electrolytes based on amorphous poly (ether−ester) s containing 1, 4, 7-trioxanonyl main chain units. Ionic conductivity versus polymer chain mobility. Macromolecules 32(10):3314–3324
Uma T, Mahalingam T, Stimming U (2005) Conductivity studies on poly(methyl methacrylate)–Li2SO4 polymer electrolyte systems. Mater Chem Phys 90(2-3):245–249
Wagner JB, Wagner CJ (1957) Electrical conductivity measurements on cuprous halides. Chem Rev 26:1597–1601
Winnie T, Arof AK (2006) Transport properties of hexanoyl chitosan-based gel electrolyte. Ionics 12(2):149–152
Tian Khoon L, Ataollahi N, Hassan NH, Ahmad A (2016) Studies of porous solid polymeric electrolytes based on poly (vinylidene fluoride) and poly (methyl methacrylate) grafted natural rubber for applications in electrochemical devices. J Solid State Electrochem 20(1):203–213
Kadir MFZ, Majid SR, Arof AK (2010) Plasticized-chitosan-PVA blend polymer electrolyte based proton battery. Electrochim Acta 55(4):1475–1482
Mishra K, Rai DK (2013) Studies of a plasticized PEO+NH4PF6 proton-conducting polymer electrolyte system and its application in a proton battery. J Korean Phys Soc 62(2):311–319
Monisha S, Mathavan T, Selvasekarapandian S, Beniala AMF, Aristatilc G, Manic N, Premalatha M, Vinoth pandi D (2017) Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr Polym 157:38–47
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moniha, V., Alagar, M., Selvasekarapandian, S. et al. Synthesis and characterization of bio-polymer electrolyte based on iota-carrageenan with ammonium thiocyanate and its applications. J Solid State Electrochem 22, 3209–3223 (2018). https://doi.org/10.1007/s10008-018-4028-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-4028-6