Abstract
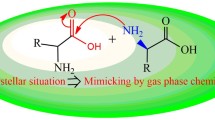
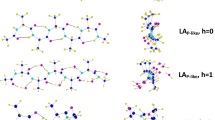
The conformational preferences of three azadipeptides Ac-Pro-azaXaa-NHMe [Xaa = Asn (1), Asp (2), Ala (3)] have been carried out in gas phase and solution (water) using the density functional method B3LYP/6–311 + + G(d,p) to explore the effect of the change of side chain of azaamino acids at the i + 2 position on the stability of these components. The most stable conformations of compounds (1), (2), and (3) have an amid bond oriented trans, trans, and cis, respectively, in gas phase, whereas the orientation of amid bond in water solvent of compounds (2) and (3) has changed to cis and trans, respectively. We have also noticed the importance of backbone-side chain hydrogen bonds in the stabilization of the β turn motif in gas phase since this motif is more stable in the case of compounds (1) and (2) and less stable in the case of compound (3) in which these hydrogen bonds are absent. Furthermore, the βII(βII′) turn structure is more stable than βI turn for all conformations of the three compounds in gas phase, while it is not true in the case of some conformations in solution. Moreover, the stability of β turn increases from azaAsn to azaAsp which could be due to the side chain’s basic nature of azaAsn. In general, hydrogen bonds were found to play a key role in the stabilization of these compounds since most of conformers are lower in energy when they have more than two hydrogen bond interactions while conformations with one or no hydrogen bonds are higher in energy and thus less stable.






Similar content being viewed by others
References
Hess HJ, Moreland WT, Laubach GD (1963) N-[2-isopropyl-3-(-aspartyl-l-arginyl)-carbazoyl]-l-tyrosyl-l-valyl-l-histidyl-l-prolyl-l-phenylalanine, an isostere of bovine angiotensin II. J Am Chem Soc 85:4040–4041
Begum A, Sujatha D, Prasad KVSRG, Bharathi K (2017) A review on azapeptides: the promising peptidomimetics. Asian J Chem 29:1879–1887
Lv Z, Chu Y, Wang Y (2015) HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV/AIDS - Res Palliat Care 7:95–104
Boeglin D, Hamdan FF, Melendez RE, Cluzeau J, Laperriere A, Héroux M, Bouvier M, Lubell WD (2007) Calcitonin gene-related peptide analogues with aza and indolizidinone amino acid residues reveal conformational requirements for antagonist activity at the human calcitonin gene-related peptide 1 receptor. J Med Chem 50:1401–1408
Proulx C, Picard E, Boeglin D, Pohankova P, Chemtob S, Ong H, Lubell WD (2012) Azapeptide analogues of the growth hormone releasing peptide 6 as cluster of differentiation 36 receptor ligands with reduced affinity for the growth hormone secretagogue receptor 1a. J Med Chem 55:6502–6511
Ro S, Lee HJ, Ahn IA, Shin DK, Lee KB, Yoon CJ, Choi YS (2001) Torsion angle based design of peptidomimetics: a dipeptidic template adopting β-I turn (Ac-Aib-AzGly-NH2). Bioorganic Med Chem 9:1837–1841
Lee HJ, Ahn IA, Ro S, Choi KH, Choi YS, Lee KB (2000) Role of azaamino acid residue in β-turn formation and stability in designed peptide. J Pept Res 56:35–46
Lee HJ, Song JW, Choi YS, Ro S, Yoon CJ (2001) The energetically favorable cis peptide bond for the azaglycine-containing peptide: For-AzGly-NH2 model. Phys Chem Chem Phys 3:1693–1698
Ro S, Yoon CJ (2000) Conformational preference of acetyl-azaalanine-N-methyl amide. Zeitschrift fur Phys Chemie 214:1699–1706
Lee HJ, Choi KH, Ahn IA, Ro S, Jang HG, Choi YS, Lee KB (2001) The β-turn preferential solution conformation of a tetrapeptide containing an azaamino acid residue. J Mol Struct 569:43–54
Lee HJ, Park HM, Lee KB (2007) The β-turn scaffold of tripeptide containing an azaphenylalanine residue. Biophys Chem 125:117–126
Lee HJ, Jung HJ, Kim JH, Park HM, Lee KB (2003) Conformational preference of azaglycine-containing dipeptides studied by PCM and IPCM methods. Chem Phys 294:201–210
Stewart DE, Sarkar A, Wampler JE (1990) Occurrence and role of cis peptide bonds in protein structures. J Mol Biol 214:253–260
Kang YK, Byun BJ (2007) Conformational preferences and cis–trans isomerization of azaproline residue. J Phys Chem B 111:5377–5385
Andre F, Vicherat A, Boussard G, Aubry A, Marraud M (1997) Aza-peptides III: experimental structural analysis of aza-alanine and aza-asparagine containing peptides. J Peptide Res 50:372–381
Andre F, Boussard G, Bayeul D, Didierjean C, Aubry A, Marraud M (1997) Aza-peptides. J Peptide Res 49:556–562
Andre F, Marraud M, Boussard G, Didierjean C, Aubry A (1996) Synthesis and structure of AzAsx-Pro-containing aza-Peptides. Tetrahedron Lett 37:183–186
Benatalah Z, Aubry A, Boussard G, Marraud M (1991) Evidence for a β-turn in an azadipeptide sequence”. Int J Pept Protein Res 38:603–605
Mitchell JBO, Price SL (1990) The nature of the N-H…O=C hydrogen bond: an intermolecular perturbation theory study of the formamide/formaldehyde complex. J Comput Chem 11:1217–1233
Avbelj F, Luo P, Baldwin RL (2000) Energetics of the interaction between water and the helical peptide group and its role in determining helix propensities. Proc Natl Acad Sci USA 97:10786–10791
Pace CN, Scholtz JM, Grimsley GR (2014) Forces stabilizing proteins. FEBS Lett 588:2177–2184
Ranade SS, Ramalingam R (2020) Hydrogen bonds in anoplin peptides aid in identification of a structurally stable therapeutic drug scaffold. J Mol Model 26:1–13
Fleming PJ, Rose GD (2005) Do all backbone polar groups in proteins form hydrogen bonds? Protein Sci 14:1911–1917
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT
Karplus PA (1996) Experimentally observed conformation-dependent geometry and hidden strain in proteins. Protein Sci 5:1406–1420
Alema C, Puiggali J (1997) Conformational preferences of the asparagine residue. Gas-phase, aqueous solution, and chloroform solution calculations on the model dipeptide. J Phys Chem B 5647:3441–3446
Kang YK, Park HS (2014) Assessment of CCSD(T), MP2, DFT-D, CBS-QB3, and G4(MP2) methods for conformational study of alanine and proline dipeptides. Chem Phys Lett 600:112–117
Perczel A, McAllister MA, Császár P, Csizmadia IG (1993) Peptide models 6. New β-turn conformations from ab initio calculations confirmed by x-ray data of proteins. J Am Chem Soc 115:4849–4858
Marraud M, Aubry A (1996) Crystal structures of peptides and modified peptides. Pept Sci 40:45–83
Mcharfi M, Aubry A, Boussard G, Marraud M (1986) Backbone side-chain interactions in peptides IV. β-Turn conformations of Asp and Asp-containing dipeptides in solute and solid states. Eur Biophys J 14:43–51
Zimmerman SS, Pottle MS, Nbmethy G, Scheraga HA (1977) Macromolecules 10:1–9
Funding
There has been no significant financial support for this work that could have influenced its outcome.
Author information
Authors and Affiliations
Contributions
MEK: performed the calculations, contributed to the interpretation of the results, and wrote the manuscript. HL and ZEA: contributed to the interpretation of the results. MM and MB: supervised the findings of this work, contributed to the interpretation of the results, and were in charge of overall direction and planning. AF and ATB: contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Khabchi, M., Lahlou, H., El Adnani, Z. et al. Conformational preferences of Ac-Pro-azaXaa-NHMe (Xaa = Asn, Asp, Ala) and the effect of intramolecular hydrogen bonds on their stability in gas phase and solution. J Mol Model 27, 368 (2021). https://doi.org/10.1007/s00894-021-04992-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04992-x