Abstract
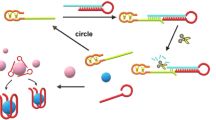
According to aptamer-mediated hairpin DNA cascade amplifier and gold nanoparticles aggregation, an optical platform for cancer cells determination has been proposed. High-affinity chimeric aptamers were used for cancer cell detection and also as an initiator for beginning hairpin assembly to construct three-way junction (3WJ) nanostructures. These three hairpins were modified at 3′ ends with biotin. In the presence of target cell, chimeric aptamer binds to its ligand on cell surface and initiates 3WJ nanostructures formation. These 3WJ nanostructures interact with streptavidin-modified gold nanoparticles (AuNPs) via non-covalent biotin-streptavidin interactions and create a crossover lattice of nanoparticles. This event leads to AuNPs aggregation and red-shifting. The results were confirmed by gel electrophoresis and UV-visible spectrophotometry. The dynamic range of this assay is 25 to 107 cells with a detection limit of 10 cells which is respectively 9 and 4 times more significant than the sensitivity of AuNP-based approaches without amplification and enzyme-mediated signal amplification.

Graphical abstract





Similar content being viewed by others
References
Wang K, Fan D, Liu Y, Wang E (2015) Highly sensitive and specific colorimetric detection of cancer cells via dual-aptamer target binding strategy. Biosens Bioelectron 73:1–6. https://doi.org/10.1016/j.bios.2015.05.044
Wild CP (2019) The global cancer burden: necessity is the mother of prevention. Nat Rev Cancer 19(3):123–124. https://doi.org/10.1038/s41568-019-0110-3
Srivastava S, Koay EJ (2019) Cancer overdiagnosis: a biological challenge and clinical dilemma. 19 (6):349-358. doi:https://doi.org/10.1038/s41568-019-0142-8
Ravan H (2015) Translating nucleic-acid hybridization into universal DNA-reporter sequences. TrAC Trends Anal Chem 65:97–106
Pirsaheb M, Mohammadi S (2019) Functionalized fluorescent carbon nanostructures for targeted imaging of cancer cells: a review. 186 (4):231. doi:https://doi.org/10.1007/s00604-019-3338-4
Liu Q, ** C, Wang Y, Fang X, Zhang X, Chen Z, Tan W (2014) Aptamer-conjugated nanomaterials for specific cancer cell recognition and targeted cancer therapy. NPG Asia Mater 6(4):e95
Yang D, Hartman MR, Derrien TL, Hamada S, An D, Yancey KG, Cheng R, Ma M, Luo D (2014) DNA materials: bridging nanotechnology and biotechnology. Acc Chem Res 47(6):1902–1911
Sha L, Zhang X, Wang G (2016) A label-free and enzyme-free ultra-sensitive transcription factors biosensor using DNA-templated copper nanoparticles as fluorescent indicator and hairpin DNA cascade reaction as signal amplifier. Biosens Bioelectron 82:85–92
Ravan H (2016) Implementing a two-layer feed-forward catalytic DNA circuit for enzyme-free and colorimetric detection of nucleic acids. Anal Chim Acta 910:68–74
Fozooni T, Ravan H, Sasan H (2017) Signal amplification technologies for the detection of nucleic acids: from cell-free analysis to live-cell imaging. Appl Biochem Biotechnol 183(4):1224–1253
Feng C, Zhu J, Sun J, Jiang W, Wang L (2015) Hairpin assembly circuit-based fluorescence cooperative amplification strategy for enzyme-free and label-free detection of small molecule. Talanta 143:101–106
Zhu G, Zhang S, Song E, Zheng J, Hu R, Fang X, Tan W (2013) Building fluorescent DNA nanodevices on target living cell surfaces. Angew Chem Int Ed 52(21):5490–5496
Zhou G, Lin M, Song P, Chen X, Chao J, Wang L, Huang Q, Huang W, Fan C, Zuo X (2014) Multivalent capture and detection of cancer cells with DNA nanostructured biosensors and multibranched hybridization chain reaction amplification. Anal Chem 86(15):7843–7848
Jie G, Jie G (2016) Sensitive electrochemiluminescence detection of cancer cells based on a CdSe/ZnS quantum dot nanocluster by multibranched hybridization chain reaction on gold nanoparticles. RSC Adv 6(29):24780–24785
Zhang X, Chen B, He M, Wang H, Hu B (2016) Gold nanoparticles labeling with hybridization chain reaction amplification strategy for the sensitive detection of HepG2 cells by inductively coupled plasma mass spectrometry. Biosens Bioelectron 86:736–740
Yin P, Choi HM, Calvert CR, Pierce NA (2008) Programming biomolecular self-assembly pathways. Nature 451(7176):318
Zhao J, **g P, Xue S, Xu W (2017) Dendritic structure DNA for specific metal ion biosensor based on catalytic hairpin assembly and a sensitive synergistic amplification strategy. Biosens Bioelectron 87:157–163
Wu Z, Fan H, Satyavolu NSR, Wang W, Lake R, Jiang JH, Lu Y (2017) Imaging endogenous metal ions in living cells using a DNAzyme–catalytic hairpin assembly probe. Angew Chem Int Ed 56(30):8721–8725
Zang Y, Lei J, Ling P, Ju H (2015) Catalytic hairpin assembly-programmed porphyrin–DNA complex as photoelectrochemical initiator for DNA biosensing. Anal Chem 87(10):5430–5436
Wu H, Liu Y, Wang H, Wu J, Zhu F, Zou P (2016) Label-free and enzyme-free colorimetric detection of microRNA by catalyzed hairpin assembly coupled with hybridization chain reaction. Biosens Bioelectron 81:303–308
Song W, Zhang Q, Sun W (2015) Ultrasensitive detection of nucleic acids by template enhanced hybridization followed by rolling circle amplification and catalytic hairpin assembly. Chem Commun 51(12):2392–2395
Li B, Chen X, Ellington AD (2012) Adapting enzyme-free DNA circuits to the detection of loop-mediated isothermal amplification reactions. Anal Chem 84(19):8371–8377
Jiang Y, Li B, Milligan JN, Bhadra S, Ellington AD (2013) Real-time detection of isothermal amplification reactions with thermostable catalytic hairpin assembly. J Am Chem Soc 135(20):7430–7433
Bi S, Yue S, Wu Q, Ye J (2016) Triggered and catalyzed self-assembly of hyperbranched DNA structures for logic operations and homogeneous CRET biosensing of microRNA. Chem Commun 52(31):5455–5458
Ravan H, Amandadi M, Esmaeili-Mahani S (2017) DNA domino-based nanoscale logic circuit: a versatile strategy for ultrasensitive multiplexed analysis of nucleic acids. Anal Chem 89(11):6021–6028
Zhou W, Liang W, Li X, Chai Y, Yuan R, **ang Y (2015) MicroRNA-triggered, cascaded and catalytic self-assembly of functional “DNAzyme ferris wheel” nanostructures for highly sensitive colorimetric detection of cancer cells. Nanoscale 7(19):9055–9061
He H, Dai J, Duan Z, Meng Y, Zhou C, Long Y, Zheng B, Du J, Guo Y, **ao D (2016) Target-catalyzed autonomous assembly of dendrimer-like DNA nanostructures for enzyme-free and signal amplified colorimetric nucleic acids detection. Biosens Bioelectron 86:985–989
Bi S, **u B, Ye J, Dong Y (2015) Target-catalyzed DNA four-way junctions for CRET imaging of microRNA, concatenated logic operations, and self-assembly of DNA nanohydrogels for targeted drug delivery. ACS Appl Mater Interfaces 7(41):23310–23319
Ravan H (2016) Isothermal RNA detection through the formation of DNA concatemers containing HRP-mimicking DNAzymes on the surface of gold nanoparticles. Biosens Bioelectron 80:67–73
Li X, Chen B, He M, Hu B (2019) Immunodetection and counting of circulating tumor cells (HepG2) by combining gold nanoparticle labeling, rolling circle amplification and ICP-MS detection of gold. 186 (6):344. doi:https://doi.org/10.1007/s00604-019-3476-8
Zhao Y, Xu D, Tan W (2017) Aptamer-functionalized nano/micro-materials for clinical diagnosis: isolation, release and bioanalysis of circulating tumor cells. Integr Biol 9(3):188–205
Shi K, Dou B, Yang J, Yuan R, **ang Y (2017) Target-triggered catalytic hairpin assembly and TdT-catalyzed DNA polymerization for amplified electronic detection of thrombin in human serums. Biosens Bioelectron 87:495–500
Kashanian S, Rafipour R, Tarighat F, Ravan H (2012) Immobilization of cobaltferritin onto gold electrode based on self-assembled monolayers. IET Nanobiotechnol 6(3):102–109
Zhang X, **ao K, Cheng L, Chen H, Liu B, Zhang S, Kong J (2014) Visual and highly sensitive detection of cancer cells by a colorimetric aptasensor based on cell-triggered cyclic enzymatic signal amplification. Anal Chem 86(11):5567–5572
Ravan H, Yazdanparast R (2012) Development of a new loop-mediated isothermal amplification assay for prt (rfbS) gene to improve the identification of Salmonella serogroup D. World J Microbiol Biotechnol 28(5):2101–2106
Ravan H, Amandadi M, Sanadgol N (2016) A highly specific and sensitive loop-mediated isothermal amplification method for the detection of Escherichia coli O157: H7. Microb Pathog 91:161–165
Medley CD, Smith JE, Tang Z, Wu Y, Bamrungsap S, Tan W (2008) Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells. Anal Chem 80(4):1067–1072
Shangguan D, Li Y, Tang Z, Cao ZC, Chen HW, Mallikaratchy P, Sefah K, Yang CJ, Tan W (2006) Aptamers evolved from live cells as effective molecular probes for cancer study. Proc Natl Acad Sci 103(32):11838–11843
Liang L, Lan F, Li L, Ge S, Yu J, Ren N, Liu H, Yan M (2016) Paper analytical devices for dynamic evaluation of cell surface N-glycan expression via a bimodal biosensor based on multibranched hybridization chain reaction amplification. Biosens Bioelectron 86:756–763
Jie G, Wang L, Yuan J, Zhang S (2011) Versatile electrochemiluminescence assays for cancer cells based on dendrimer/CdSe–ZnS–quantum dot nanoclusters. Anal Chem 83(10):3873–3880
Funding
This work was supported by Shahid Bahonar University of Kerman, Kerman, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 196 kb)
Rights and permissions
About this article
Cite this article
Ravan, H., Norouzi, A., Sanadgol, N. et al. Colorimetric nanoplatform for visual determination of cancer cells via target-catalyzed hairpin assembly actuated aggregation of gold nanoparticles. Microchim Acta 187, 392 (2020). https://doi.org/10.1007/s00604-020-04368-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04368-7