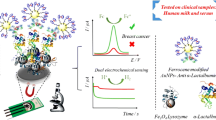
Abstract
The authors describe an electrochemical sensor for the breast cancer marker α-lactalbumin (αLA). It is based on the use of printed single-walled carbon nanotube (SWCNT) electrodes that were modified with polycatechol. Impedance-derived electrochemical capacitance spectroscopy (ECS) is applied for detection at an applied potential of −0.14 V vs. Ag/AgCl reference electrode. The electrode was prepared in a two-step process. First, a dispersion of SWCNTs was drop-cast onto the surface of a poly(ethylene terephthalate) substrate to act as the working electrode. Next, catechol was electrochemically polymerized on the SWCNTs, prior to the immobilization of lysozyme. The strong interaction between lysozyme and αLA induced changes in the redox capacitance which are detected by ECS. The latter shows the device to be capable of detecting αLA in the 20 to 80 ng·mL−1 concentration range. The limit of detection is 9.7 ng·mL−1 at an S/N ratio of 3. The device was used to detect αLA in human blood serum with good recovery results.

A sensitive biosensor for αLA was prepared by modifying SWCNT electrode with polycatechol and lysozyme. The electrochemical capacitance spectroscopy was used for the first time to selectively detect αLA in the blood in the range from 20 to 80 ng·mL−1.




Similar content being viewed by others
References
Sagadevan S, Periasamy M (2014) Recent trends in nanobiosensors and their applications-a review. Rev Adv Mater Sci 36:62
Yang N, Chen X, Ren T, Zhang P, Yang D (2015) Carbon nanotube based biosensors. Sensors Actuators B 207:690
Kumar S, Ahlawat W, Kumar R, Dilbaghi N (2015) Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of nanobiosensors for healthcare. Biosens Bioelectron 70:498
Jia L, Lawrence G, Balasubranian VV, Choi G, Choy JH, Abdullah AM, Elzatahry A, Ariga K, Vinu A (2015) Highly ordered nanoporous carbon films with tunable pore diameters and their excellent sensing properties. Chem Eur J 21:697
DeSantis C, Ma J, Bryan L, Jemal A (2014) Breast cancer statistics, 2013. CA Cancer J Clin 64:52
Hayes DF (2016) Is breast cancer a curable desease? J Oncol Practice 12:13
Azimzadeh M, Rahaiea M, Nasirizadeh N, Ashtari K, Manesh HN (2016) An electrochemical nanobiosensor for plasma miRNA-155, based on graphene oxide and gold nanorod, for early detection of breast cancer. Biosens Bioelectron 77:99
Weigel MT, Dowsett M (2010) Current and emerging biomarkers in breast cancer: prognosis and prediction. Enodcr Relat Cancer 17:245
Zhang SJ, Hu Y, Qian HL, Jiao SC, Liu ZF, Tao HT, Han L (2013) Expression and significance of ER, PR, VEGF, CA15-3, CA125 and CEA in judging the prognosis of breast cancer. Asian Pac J Cancer Prev 14:3937
Tabasi A, Noorbakhsh A, Sharifi S (2017) Reduced graphene oxide-chitosan-aptamer interface as new platform for ultrasensitive detection of human epidermal growth factor receptor 2. Biosens Bioelectron 95:117
Botelho ACF, Ferreira MC, França JL, França EL, França ACH (2012) Breastfeeding and its relationship with reduction of breast cancer: a review. Asian Pac J Cancer Prev 13:5327
Thean ET, Toh BH (1990) Serum human alpha-lactalbumin as a marker for breast cancer. Br J Cancer 61:773
Nigen M, Croguennec T, Renard D, Bouhallab S (2007) Temperature affects the supramolecular structures resulting from α-lactalbumin-lysozyme interaction. Biochemistry 46:1248
Qasba PK, Kumar S (1997) Molecular divergence of lysozymes and α-lactalbumin. Crit Rev Biochem Mol Biol 32:255
Yang A, Zheng Y, Long C, Chen H, Liu B, Li X, Yuan J, Cheng F (2014) Fluorescent immunosorbent assay for the detection of alpha lactalbumin in dairy products with monoclonal antibody bioconjugated with CdSe/ZnS quantum dots. Food Chem 150:73
Santos A, Carvalho FC, Barreira MCR, Bueno PR (2014) Impedance-derived electrochemical capacitance spectroscopy for the evaluation of lectin-glycoprotein binding affinity. Biosens Bioelectron 62:102
Fernandes FCB, Santos A, Martins DC, Góes MS, Bueno PR (2014) Comparing label free electrochemical impedimetric and capacitive biosensing architectures. Biosens Bioelectron 57:96
Rabti A, Martinez CCM, Pires LB, Raouafi N, Merkoçi A (2016) Ferrocene-functionalized graphene electrode for biosensing applications. Anal Chim Acta 926:28
Rabti A, Argoubi W, Raouafi N (2016) Enzymatic sensing of glucose in artificial saliva using a flat electrode consisting of a nanocomposite prepared from reduced graphene oxide, chitosan, nafion and glucose oxidase. Microchim Acta 183:1227
Tominaga M, Sasaki A, Togami M (2016) Bioelectrocatalytic oxygen reaction and chloride inhibition resistance of laccase immobilized on single-walled carbon nanotube and carbon paper electrodes. Electrochemisry 84:315
Eguílaz M, Gutierrez F, Domínguez JMG, Martínez MT, Rivas G (2016) Single-walled carbon nanotubes covalently functionalized with polytyrosine: a new material for the development of NADH-based biosensors. Biosens Bioelectron 86:308
Stojanović ZS, Mehmeti E, Kalcher K, Guzsvány V, Stanković DM (2016) SWCNT-modified carbon paste electrode as an electrochemical sensor for histamine determination in alcoholic beverages. Food Anal Methods 9:2701
Lew W, Dai L (2010) Carbon nanotube supercapacitors. In: Marulanda JM (ed) Carbon nanotubes, 1st edn. InTech, Rijeka, pp 563–593
Adhikari BR, Schraft H, Chen A (2017) High-performance enzyme entrapment platform facilitated by a cationic polymer for the efficient electrochemical sensing of ethanol. Analyst 142:2595
Pagán M, Suazo D, Toro N, Griebenow K (2015) A comparative study of different protein immobilization methods for the construction of an efficient nano-structured lactate oxidase-SWCNT-biosensor. Biosens Bioelectron 64:138
Snow ES, Perkins FK (2005) Capacitance and conductance of single-walled carbon nanotubes in the presence of chemical vapors. Nano Lett 15:2414
**ao H, Zhu B, Wang D, Pang Y, He L, Ma X, Wang R, ** C, Chen Y, Zhu X (2012) Photodynamic effects of chlorin e6 attached to single wall carbon nanotubes through noncovalent interactions. Carbon 50:1681
Mosch HLKS, Akintola O, Plass W, Hoeppener S, Schubert US, Ignaszak A (2016) The specific surface versus electrochemically active area of the carbon/polypyrrole capacitor: the correlation of ion dynamics studied by an electrochemical quartz crystal microbalance with BET surface. Langmuir 32:4440
**ong S, Wei J, Jia P, Yang L, Ma J, Lu X (2011) Water-Processable polyaniline with covalently bonded single-walled carbon nanotubes: enhanced electrochromic properties and impedance analysis. Appl Mater Interfaces 3:782
Bai J, Bo X, Qi B, Guo L (2010) A novel Polycatechol/ordered mesoporous carbon composite film modified electrode and its Electrocataly ic application. Electroanalysis 22(15):1750
Sayyah SM, Khaliel AB, Azooz RE, Mohamed F (2011) Electropolymerization of some Ortho- substituted phenol derivatives on Pt-electrode from aqueous acidic solution; kinetics, Mechanism, electrochemical studies and characterization of the polymer obtained. In: Balcerzak ES (ed) Electropolymerization. InTech, Rijeka, pp 1–53. isbn:978-953-307-693-5
Marczewska B, Przegalinski M (2013) Poly(catechol) electroactive film and its electrochemical properties. Synth Met 182:33
Kristiansen A, Vårum KM, Grasdalen H (1998) The interactions between highly de-N-acetylated chitosans and lysozyme from chicken egg white studied by 1H-NMR spectroscopy. Eur J Biochem 251:335
Fukamizo T, Yamaguchi T, Araki T, Torikata T, Kristiansen A, Vårum KM (2001) Binding of a highly de-N-acetylated chitosan to Japanese Pheasan lysozyme as measured by 1H-NMR spectroscopy. Biosci Biotechnol Biochem 65:1766
Fernandes FCB, Góes MS, Davis JJ, Bueno PR (2013) Label free redox capacitive biosensing. Biosens Bioelectron 50:437
Lehr J, Fernandes FCB, Bueno PR, Davis JJ (2014) Label-free capacitive diagnostics: exploiting local redox probe state occupancy. Anal Chem 86:2559
Nigen M, Croguennec T, Bouhallab S (2009) Formation and stability of α-lactalbumin-lysozyme spherical particles: involvement of electrostatic forces. Food Hydrocoll 23:510
Nigen M, Gaillard C, Croguennec T, Madec M-N, Bouhallab S (2010) Dynamic and supramolecular organization of α-lactalbumin/lysozyme microspheres: microscopic study. Biophys Chem 146:30
Bueno PR, Davis JJ (2012) Elucidating redox-level dispersion and local dielectric effects within electroactive molecular films. Anal Chem 86:1997
Bueno PR, Mizzon G, Davis JJ (2012) Capacitance spectroscopy: a versatile approach to resolving the redox density of states and kinetics in redox-active self-assembled monolayers. J Phys Chem B 116:8822
Tao C, Zhang Q, Feng N, Shi D, Liu B (2016) Development of a colloidal gold immunochromatographic strip assay for simple and fast detection of human a-lactalbumin in genetically modified cow milk. J Dairy Sci 99:1773
Montiel VRV, Campuzano S, Rodríguez RMT, Reviejo AJ, **arrón JM (2016) Electrochemical magnetic beads-based immunosensing for the determination of α-lactalbumin in milk. Food Chem 213:595
Conrado LS, Veredas V, Nóbrega ES, Santana CC (2005) Concentration of α-lactalbumin from cow milk whey through expanded bed adsorption using a hydrophobic resin. Baz J Chem Eng 22:501
Acknowledgments
The authors acknowledge the financial support from the Tunisian Ministry of Higher Education and Scientific Research (for LCAE-LR99ES15 lab) and the mobility “Bourse d’Alternance” grant from the University of Tunis El Manar awarded to AR. ANEC (ISP-funded network) is also acknowledged for the mobility grant to AR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Raouafi, A., Rabti, A. & Raouafi, N. A printed SWCNT electrode modified with polycatechol and lysozyme for capacitive detection of α-lactalbumin. Microchim Acta 184, 4351–4357 (2017). https://doi.org/10.1007/s00604-017-2481-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2481-z