Abstract
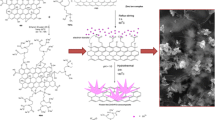
A composite consisting of chitosan containing azidomethylferrocene covalently immobilized on sheets of reduced graphene oxide was drop-casted on a polyester support to form a screen-printed working electrode that is shown to enable the determination of nitrite by cyclic voltammetry and chronoamperometry. Both reduction and oxidation of nitrite can be accomplished due to the high electron-transfer rate of this electrode. Under optimal experimental conditions (i.e. an applied potential of 0.7 V vs. Ag/AgCl in pH 7.0 solution), the calibration plot is linear in the 2.5 to 1450 μM concentration range, with an ~0.35 μM limit of detection (at a signal-to-noise ratio of 3). The sensor was successfully applied to the determination of nitrite in spiked mineral water samples, with recoveries ranging between 95 and 101 %.

We describe the design of ferrocene-functionalized reduced graphene oxide electrode and its electrocatalytic properties towards the determination of nitrite. Compared to a reduced graphene oxide electrode, the sensor exhibits enhanced electrocatalytic activity towards both oxidation and reduction of nitrite.




Similar content being viewed by others
References
Yue R, Lu Q, Zhou Y (2011) A novel nitrite biosensor based on single–layer graphene nanoplatelet–protein composite film. Biosens Bioelectron 26:4436
Huang YG, Ji JD, Hou QN (1996) A study on carcinogenesis of endogenous nitrite and nitrosamine, and prevention of cancer. Mutat Res Fundam Mol Mech Mutagen 358:7
Mirvish SS (1995) Role of N–nitroso compounds (NOC) and N–nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett 93:17
Tabacova S, Balabaeva L, Little RE (1997) Maternal exposure to exogenous nitrogen–compounds and complications of pregnancy. Arch Environ Health 52:341
Greer FR, Shannon M (2005) The committee on nutrition, and the committee on environmental health, infant methemoglobinemia: the role of dietary nitrate in food and water. Pediatrics 116:784
Zhao K, Song H, Zhuang S, Dai L, He P, Fang Y (2007) Determination of nitrite with the electrocatalytic property to the oxidation of nitrite on thionine modified aligned carbon nanotubes. Electrochem Commun 9:65
Dreysea P, Isaacs M, Calfumán K, Cáceres C, Aliaga A, Aguirre MJ, Villagra D (2011) Electrochemical reduction of nitrite at poly–[Ru(5–NO2–phen)2Cl] tetrapyridylporphyrin glassy carbon modified electrode. Electrochim Acta 56:5230
Chinthaginjala JK, Villa A, DS S, Mojet BL, Lefferts L (2012) Nitrite reduction over Pd supported CNFs: metal particle size effect on selectivity. Catal Today 183:119
Kozu BR, Rees NV, Compton RG (2010) Electrochemical determination of nitrite at a bare glassy carbon electrode; why chemically modify electrodes? Sensors Actuators B 143:539
Xu G, Liang S, Fan J, Sheng G, Luo X (2016) Amperometric sensing of nitrite using a glassy carbon electrode modified with a multilayer consisting of carboxylated nanocrystalline cellulose and poly(diallyldimethyl ammonium) ions in a PEDOT host. Microchim Acta 183:2031
Lin P, Chai F, Zhang R, Xu G, Fan X, Luo X (2016) Electrochemical synthesis of poly(3,4-ethylenedioxythiophene) doped with gold nanoparticles, and its application to nitrite sensing. Microchim Acta 183:1235
Wang J, Zhou H, Fan D, Zhao D, Xu C (2015) A glassy carbon electrode modified with nanoporous PdFe alloy for highly sensitive continuous determination of nitrite. Microchim Acta 182:1055
Huang S-S, Liu L, Mei L-P, Zhou J-Y, Guo F-Y, Wang A-J, Feng J-J (2016) Electrochemical sensor for nitrite using a glassy carbon electrode modified with gold-copper nanochain networks. Microchim Acta 183:791
Dai J, Deng D, Yuan Y, Zhang J, Deng F, He S (2016) Amperometric nitrite sensor based on a glassy carbon electrode modified with multi-walled carbon nanotubes and poly(toluidine blue. Microchim Acta 183:1553
Mehmeti E, Stanković DM, Hajrizi A, Kalcher K (2016) The use of graphene nanoribbons as efficient electrochemical sensing material for nitrite determination. Talanta 159:34
Mani V, Periasamy AP, Chen SM (2012) Highly selective amperometric nitrite sensor based on chemically reduced graphene oxide modified electrode. Electrochem Commun 17:75
Liu M, Wang L, Meng Y, Chen Q, Li H, Zhang Y, Yao S (2014) (4–ferrocenylethyne) phenylamine functionalized graphene oxide modified electrode for sensitive nitrite sensing. Electrochim Acta 116:504
Li XR, Liu J, Kong FY, Liu XC, JJ X, Chen HY (2012) Potassium–doped graphene for simultaneous determination of nitrite and sulfite in polluted water. Electrochem Commun 20:109
Ye D, Luo L, Ding Y, Chen Q, Liu X (2011) A novel nitrite sensor based on graphene/polypyrrole/chitosan nanocomposite modified glassy carbon electrode. Analyst 136:4563
Xu F, Deng M, Liu Y, Ling X, Deng X, Wang L (2014) Facile preparation of poly(diallyldimethylammonium chloride) modified reduced graphene oxide for sensitive detection of nitrite. Electrochem Commun 47:33
Marlinda AR, Pandikumar A, Yusoff N, Huang NM, Lim HN (2015) Electrochemical sensing of nitrite using a glassy carbon electrode modified with reduced functionalized graphene oxide decorated with flower-like zinc oxide. Microchim Acta 182:1113
Stankovic DM, Mehmeti E, Zavasnik J, Kalcher K (2016) Determination of nitrite in tap water: a comparative study between cerium, titanium and selenium dioxide doped reduced graphene oxide modified glassy carbon electrodes. Sensors Actuators B 236
Rabti A, Raouafi N, Merkoçi A (2016) Bio(sensing) devices based on ferrocene–functionalized graphene and carbon nanotubes. Carbon 108:481
Rabti A, Argoubi W, Raouafi N (2016) Enzymatic sensing of glucose in artificial saliva using a flat electrode consisting of a nanocomposite prepared from reduced graphene oxide, chitosan, nafion and glucose oxidase. Microchim Acta 183(3):1227
Rabti A, Mayorga-Martinez CC, Baptista-Pires L, Raouafi N, Merkoçi A (2016) Ferrocene-functionalized graphene electrode for biosensing applications. Anal Chim Acta 926:28
Barbero C, Miras MC, Calvo EJ, Kötz R, Haas O (2002) A probe beam deflection study of ion exchange at poly(vinylferrocene) films in aqueous and nonaqueous electrolytes. Langmuir 18:2756
Tang Y, Zeng X (2008) Poly(vinylferrocene) redox behavior in ionic liquids. J Electrochem Soc 155:F82
Crumbliss AL, Cooke JD, Castillo J, Wisian–Neilson P (1993) Redox properties of phosphazene polymers with pendant ferrocene groups. Inorg Chem 32:6088
Whitman LR, Bork KP, Tang Y (2011) Two–dimensional correlation in cyclic voltammetry and electrochemical quartz crystal microbalance: a complementary tool to conventional techniques. J Electroanal Chem 661:100
Kandimalla VB, Tripathi VS, HX J (2006) A conductive ormosil encapsulated with ferrocene conjugate and multiwall carbon nanotubes for biosensing application. Biomaterials 27:1167
Fabre B, Samorì C, Bianco A (2012) Immobilization of double functionalized carbon nanotubes on glassy carbon electrodes for the electrochemical sensing of the biotin–avidin affinity. J Electroanal Chem 665:90
Crumbliss AL, Cooke JD, Castillo J, Wisian–Neilson P (1993) Redox properties of phosphazene polymers with pendant ferrocene groups. Inorg Chem 32:6088
Radhakrishnan S, Krishnamoorthy K, Sekar C, Wilson J, Kima SJ (2014) A highly sensitive electrochemical sensor for nitrite detection based on Fe2O3 nanoparticles decorated reduced graphene oxide nanosheets. Appl Catal B 148–149:22
Zhao YD, Zhang WD, Luo QM, Li SFY (2003) The oxidation and reduction behavior of nitrite at carbon nanotubes powder microelectrodes. Microchem J 75:189
Cui L, Zhu J, Meng X, Yin H, Pan X, Ai S (2012) Controlled chitosan coated Prussian blue nanoparticles with the mixture of graphene nanosheets and carbon nanoshperes as a redox mediator for the electrochemical oxidation of nitrite. Sensors Actuators B 161:641
Soderberg JN, Co AC, Sirk AHC, Birss VI (2006) Impact of porous electrode properties on the electrochemical transfer coefficient. J Phys Chem B 110:10401
Kalcher K (1986) A new method for the voltammetric determination of nitrite. Talanta 33:489
Acknowledgments
A. Rabti acknowledges the support given by the University of Tunis El Manar and the LCAE laboratory (LR99ES15) for research and travel funds. NR acknowledges the support from TWAS for the research grant (13-413 RG/PHA/AF/AC_C).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 594 kb)
Rights and permissions
About this article
Cite this article
Rabti, A., Ben Aoun, S. & Raouafi, N. A sensitive nitrite sensor using an electrode consisting of reduced graphene oxide functionalized with ferrocene. Microchim Acta 183, 3111–3117 (2016). https://doi.org/10.1007/s00604-016-1959-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1959-4