Abstract
Purpose
Symptomatic lumbar spinal stenosis can be treated with decompression surgery. A recent review reported that, after decompression surgery, 1.6–32.0% of patients develop postoperative symptomatic spondylolisthesis and may therefore be indicated for lumbar fusion surgery. The latter can be more challenging due to the altered anatomy and scar tissue. It remains unclear why some patients get recurrent neurological complaints due to postoperative symptomatic spondylolisthesis, though some associations have been suggested. This study explores the association between key demographic, biological and radiological factors and postoperative symptomatic spondylolisthesis after lumbar decompression.
Methods
This retrospective cohort study included patients who had undergone lumbar spinal decompression surgery between January 2014 and December 2016 at one of two Spine Centres in the Netherlands or Switzerland and had a follow-up of two years. Patient characteristics, details of the surgical procedure and recurrent neurological complaints were retrieved from patient files. Preoperative MRI scans and conventional radiograms (CRs) of the lumbar spine were evaluated for multiple morphological characteristics. Postoperative spondylolisthesis was evaluated on postoperative MRI scans. For variables assessed on a whole patient basis, patients with and without postoperative symptomatic spondylolisthesis were compared. For variables assessed on the basis of the operated segment(s), surgical levels that did or did not develop postoperative spondylolisthesis were compared. Univariable and multivariable logistic regression analyses were used to identify associations with postoperative symptomatic spondylolisthesis.
Results
Seven hundred and sixteen patients with 1094 surgical levels were included in the analyses. (In total, 300 patients had undergone multilevel surgery.) ICCs for intraobserver and interobserver reliability of CR and MRI variables ranged between 0.81 and 0.99 and 0.67 and 0.97, respectively. In total, 66 of 716 included patients suffered from postoperative symptomatic spondylolisthesis (9.2%). Multivariable regression analyses of patient-basis variables showed that being female [odds ratio (OR) 1.2, 95%CI 1.07–3.09] was associated with postoperative symptomatic spondylolisthesis. Higher BMI (OR 0.93, 95%CI 0.88–0.99) was associated with a lower probability of having postoperative symptomatic spondylolisthesis.
Multivariable regression analyses of surgical level-basis variables showed that levels with preoperative spondylolisthesis (OR 17.30, 95%CI 10.27–29.07) and the level of surgery, most importantly level L4L5 compared with levels L1L3 (OR 2.80, 95%CI 0.78–10.08), were associated with postoperative symptomatic spondylolisthesis; greater facet joint angles (i.e. less sagittal-oriented facets) were associated with a lower probability of postoperative symptomatic spondylolisthesis (OR 0.97, 95%CI 0.95–0.99).
Conclusion
Being female was associated with a higher probability of having postoperative symptomatic spondylolisthesis, while having a higher BMI was associated with a lower probability. When looking at factors related to postoperative symptomatic spondylolisthesis at the surgical level, preoperative spondylolisthesis, more sagittal orientated facet angles and surgical level (most significantly level L4L5 compared to levels L1L3) showed significant associations. These associations could be used as a basis for devising patient selection criteria, stratifying patients or performing subgroup analyses in future studies regarding decompression surgery with or without fusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lumbar spinal stenosis is associated with ageing and spinal degeneration, and can result in radiculopathy or neurogenic claudication [1]. If the stenosis is symptomatic, and when conservative interventions have failed, the surgical intervention consists of decompression surgery involving removal of (part of) the lamina, facet joints and ligaments [2]. In a recent review, Ramhmdani et al. reported that between 1.6 and 32.0% of patients develop spondylolisthesis after decompression surgery, believed to be caused by altered spinal biomechanics, leading to instability of the spinal segment(s) [2, 3]. When postoperative spondylolisthesis is associated with recurrent complaints of radiculopathy or neurogenic claudication, patients may be offered revision surgery of the affected segment in the form of a lumbar fusion [2, 4,5,6,7].
Many studies have been carried out to assess the relative effectiveness of decompression alone versus decompression with fusion in patients with lumbar spinal stenosis with or without spondylolisthesis and there is currently no evidence for the superiority of one procedure over the other [3, 5,6,7,8]. Most of the aforementioned studies have only evaluated whether there is a need for decompression to be accompanied by fusion in groups of patients whose main commonality is their having stenosis with/without spondylolisthesis; however, knowing the individual variability in the presentation of these conditions, the patients likely vary widely in their constellation of symptoms, imaging features, etc. Possibly, some patients will benefit from additional fusion, whereas others will have less need for it, but no studies have been able to clarify the circumstances under which one might consider adding a fusion during the index decompression surgery. Other than “having spondylolisthesis in addition to stenosis,” no criteria have been used (or are indeed available) to pre-stratify patients prior to randomization or to perform post hoc subgroup analyses of treatment effects based on factors that are associated with a propensity to develop post-decompression spondylolisthesis—factors that might be expected to tip the balance in favour of adding fusion during the index surgery. This is the result of the limited number of papers and mostly small non-clinical studies, reporting on determinants or correlates of post-surgical spondylolisthesis in this study population [2, 4, 5, 9,10,11].
The aim of this large, two-centre international cohort study was to explore factors associated with postoperative symptomatic spondylolisthesis after decompression surgery. The factors that were analysed included patient characteristics, surgical variables and morphological/pathological characteristics measured on preoperative MRI scans and conventional radiograms (CRs) of the lumbar spine.
Materials and methods
Patient selection
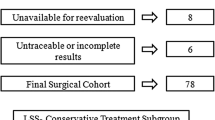
The Medical Ethical Committee at the respective hospitals approved this cohort study (METCZ20180075). Patients were identified based on healthcare codes 30,326–30,329 (one-level laminectomy or laminectomy at two levels or more) for the Dutch hospital. For the Swiss hospital, relevant fields in the local spine surgery outcomes database (which is operated within the framework of the EUROSPINE Spine Tango registry) were searched for patients that had undergone lumbar decompression surgery without fusion. Patients were included if they had undergone lumbar decompression surgery between January 2014 and December 2016 in one of the two participating centres. Exclusion criteria were: age under 18 years, no available preoperative MRI scans, scoliosis with a Cobb angle above 20°, previous fusion surgery of any lumbar segment, additional discectomy or previous decompression surgery at the level of interest, vertebral fracture at the level of interest and a follow-up of two years after decompression surgery. Follow-up data were retrieved from questionnaires, patient reports or MRI scans. Patients were actively contacted if no follow-up data were available in the patient chart regarding recurrent neurological complaints (radicular complaints or neurogenic claudication) and/or lumbar fusion surgery. The absence of postoperative symptomatic spondylolisthesis was assumed if available postoperative radiographs or MRI scans showed no postoperative spondylolisthesis, if there were no recurrent neurological complaints, and no lumbar fusion surgery had been performed in two-year follow-up.
Data collection
Based on expert opinion and the literature, the following variables were chosen to explore possible associations with postoperative symptomatic spondylolisthesis:
Patient characteristics: sex, age at the time of surgery, BMI, smoking at time of surgery (yes/no) and rheumatoid arthritis (yes/no). Variables were obtained from patient files.
Surgical variables: level of surgery (L1L2–L5S1), method of decompression surgery (interlaminar decompression or laminectomy) and surgeon performing the surgery. For example, interlaminar decompression of L4L5 is attributed to level of surgery L4L5, and laminectomy L4 is also attributed to level of surgery L4L5. Variables were obtained from surgical reports and patient files.
Morphological and pathological characteristics: Disc height, Pfirrmann grade (for definition, see below) and pre- and postoperative spondylolisthesis were measured on sagittal T2-weighted MRI scans, while facet joint angles, facet joint tropism, facet effusion, facet joint degeneration grade, cross-sectional area (CSA) and greyscale of paravertebral muscles [M. psoas, M. multifidus (MF) and M. erector spinae (ES)], CSA of vertebral body and greyscale of fat were measured on axial T2-weighted MRI images using SECTRA IDS7 (Appendix 1).
The correct slice for measurements on axial MRI scans was chosen by locating the inferior part of the intervertebral disc at the level of spinal stenosis [12, 13]. Researchers measuring preoperative morphological and pathological characteristics were blinded to the occurrence of postoperative symptomatic spondylolisthesis.
To assess intervertebral disc height, the average was taken from the anterior, middle and posterior part of the disc [14] (Appendix 1, Fig. 1). Intervertebral disc degeneration grade was visually assessed according to the Pfirrmann et al. grading scheme, classifying the intervertebral disc into five categories from 1 (normal) to 5 (collapsed disc space) [15].
To calculate facet joint angles, two lines were placed between the two peaks of the superior articular facet joint towards the centre of the vertebral body and another midsagittal line through the spinous processes. The angle between the lines was defined as facet joint angle [16,17,18] (Appendix 1, Fig. 2). The mean of the two facet joint angles was calculated and presented. Facet joint tropism was assessed with “yes” or “no” by calculating a difference between left and right facet joint angle where a difference above 10° being counted as tropism present [16, 17]. The presence of effusion was visible on axial MRI scans as a hyperintensity of ≥ 1 mm within the facet joint and was assessed with “yes” or “no” [19]. To determine the facet joint degeneration grade, the protocol of Weishaupt et al. was used, with grades being assigned between 0 (normal) and 3 (severe degeneration) [20].
For measurement of vertebral body CSA, the border of the vertebral body was marked. For muscle measurements, muscle borders of the left and right M. psoas, MF and ES were marked, avoiding fat tissue and connective tissue [16, 21,22,23] (Appendix 1, Fig. 3). The relative CSA of the muscles was calculated by dividing muscle CSA by vertebral body CSA [12, 23, 24]. Greyscale of fat tissue was measured using the method of Valentin et al. [25] (Appendix 1, Fig. 3). The relative greyscale of the muscles was calculated by dividing muscle greyscale by fat tissue greyscale [21, 22, 25, 26]. The mean of the left and right relative CSA and greyscale of the muscles was calculated.
The extent of pre- and postoperative spondylolisthesis was measured on MRI scans by placing a line along the posterior side of the lower vertebral body and a second line along the posterior side of the upper vertebral body [5] (Appendix 1, Fig. 4). Postoperative spondylolisthesis was measured on postoperative MRI scans, independently by two researchers as a continuous variable in mm. Only when both researchers identified a postoperative spondylolisthesis, and when the patient had recurrent neurological complaints, the patient was assigned to the group with postoperative symptomatic spondylolisthesis. If spondylolisthesis was present preoperatively, a worsening of any distance after decompression surgery was counted as postoperative symptomatic spondylolisthesis. This worsening was only considered real worsening if both researchers independently measured a difference in slippage.
Sagittal balance parameters [pelvic incidence (PI), sacral slope (SS), pelvic tilt (PT) and lumbar lordosis (LL)] were measured on preoperative sagittal CR of the lumbar spine using Surgimap 2.3.2.1 (Appendix 1, Fig. 5). These parameters were determined according to generally accepted definitions [27, 28].
Sample size
As a rule of thumb, ten events per variable are necessary to find associations in logistic regression models [29]. The number of variables to be included in the multivariable regression model depended on the number of independent variables with a P-value below 0.20 in the univariate regression models. Four variables were included in the multivariable regression analyses of postoperative symptomatic spondylolisthesis in patient-basis variables. This required that there be a minimum of 40 patients with and 40 patients without postoperative symptomatic spondylolisthesis. Seven variables were included in the multivariable regression analyses of surgical level-basis variables of which one variable had three subgroups. This required that there be a minimum of 80 surgical levels with and 80 surgical levels without postoperative symptomatic spondylolisthesis.
Statistical analyses
Analyses were divided into those using patient-basis variables (age, gender, BMI, smoking, rheumatoid arthritis, type of surgery, single- or multilevel surgery, PI, SS, PT and LL) and those using surgical level-basis variables (level of surgery, facet angles, facet tropism, facet joint effusion, facet degeneration, disc height, disc degeneration, preoperative spondylolisthesis, CSA and greyscale of M. psoas, MF and ES). Patient-basis variables were analysed for the total number of included patients. Surgical level-basis variables were analysed for the total number of included surgical levels, since some patients underwent multilevel decompression surgery.
For these analyses, groups were formed based on the occurrence of postoperative symptomatic spondylolisthesis. Univariate logistic regression analyses were performed for both patient-basis variables and surgical level-basis variables, to determine associations between individual factors and the presence of postoperative symptomatic spondylolisthesis. Subsequently, multivariable logistic regression was performed, including variables from the univariate logistic regression analyses with an α-value below 0.20. Variables were removed from the analysis if the α-value was above 0.05 using stepwise backward elimination. Statistical analyses were performed using IBM SPSS Statistics 25.
Reliability measurements
Intraobserver and interobserver reliability of measurements were assessed for the following continuous variables: facet joint angles, disc height, muscle CSA and greyscale, and sagittal balance parameters. A randomly chosen 10% of the measurements were taken twice by the same researcher with at least three weeks between measurements and a further random 10% were repeated by a second researcher. Data were analysed using intraclass correlation coefficients (ICC), two-way mixed analysis with absolute agreement and accepted as adequate if ICC > 0.60 (33).
Results
Patient population
A total of 1723 patients underwent lumbar decompression surgery between January 2014 and December 2016; 1186 patients were excluded from the study, mostly because they had undergone decompression surgery of the cervical or thoracic spine or received additional intervertebral disc removal (see Appendix 2 flow chart). Finally, 716 patients were included in the analysis of patient-basis variables. Single-level surgery was performed in 416 patients and multilevel surgery in 300 patients. Preoperative spondylolisthesis was present in 243 patients (33.9%). Preoperative CRs were only available for 488 patients, because they were not always part of standard care. This resulted in a total of 1094 surgical levels that could be included in the analysis of surgical level-basis variables.
Intraobserver and interobserver reliability
Assessment of intraobserver and interobserver reliability showed ICCs ranging from 0.81 to 0.99 and 0.67 to 0.97, respectively, depending on the variable in question (Table 1), all exceeding the required threshold for acceptable reliability of ICC > 0.60.
Patient characteristics and regression analyses
For the variable “level of surgery,” the categories L1L2 and L2L3 were combined to make a category L1L3, because the frequency of decompression surgery at these levels was low. Facet joints graded as degeneration grade 0 or 1 were combined as grade 0–1, because frequency of these grades was low.
First, analyses were performed on patient-basis variables. In total, 66 of 716 included patients developed postoperative symptomatic spondylolisthesis (9.2%). Univariable logistic regression showed an association between postoperative symptomatic spondylolisthesis and female sex, lower BMI and increased PI and PT (Table 2). There was no significant difference in the occurrence of postoperative symptomatic spondylolisthesis across surgeons.
Multivariable logistic regression was performed including two patient-basis variables identified from the univariate regression analysis with P-values below 0.20. Sagittal balance parameters were excluded from the multivariable analysis, since the data of 228 patients were missing. Two independent associations were found after stepwise backward elimination: patients had a higher probability of postoperative symptomatic spondylolisthesis if they were female. Higher BMI decreased the probability of having postoperative symptomatic spondylolisthesis (Table 3).
Second, analyses were performed on surgical level-basis variables. Postoperative symptomatic spondylolisthesis was recorded in 72 of 1094 (6.6%) included surgical levels. Univariate logistic regression for surgical level-basis variables showed an association between postoperative symptomatic spondylolisthesis and smaller facet joint angles (more sagittal orientated facets), presence of preoperative spondylolisthesis, surgery at level L4L5 compared to levels L1L3 and increased greyscale of MF. Lower probability of postoperative symptomatic spondylolisthesis was associated with facet degeneration grade 2 compared with grade 3 (Table 4).
After including nine surgical level-basis variables in the multivariable regression analysis, three associations were found after stepwise backward elimination: surgical levels with preoperative spondylolisthesis, smaller facet joint angles (i.e. more sagittal-oriented) and level of surgery (Table 5).
Discussion
This study aimed to explore factors associated with postoperative symptomatic spondylolisthesis after decompression surgery. A total of 716 patients, representing 1094 surgical levels, were included in the analyses. Statistical analyses were carried out separately for patient-basis variables and for surgical level-basis variables. In total, 66 of 716 included patients had postoperative symptomatic spondylolisthesis (9.2%). Female sex showed a positive association with the presence of postoperative symptomatic spondylolisthesis after decompression surgery, while increasing BMI was associated with a lower probability of spondylolisthesis. Postoperative symptomatic spondylolisthesis was observed in 72 of 1094 (6.6%) included surgical levels. On a surgical level-basis, preoperative spondylolisthesis, level of surgery (based on univariable regression analyses; most significantly level L4L5 compared to levels L1L3) and smaller facet angles (i.e. more sagittal-oriented facets) were associated with postoperative symptomatic spondylolisthesis. ICCs for intraobserver and interobserver reliability of the morphological variables, determined on preoperative MRI scans and CRs, were in the range 0.67–0.99, i.e. above the acceptable value of 0.60 for all variables.
Patient-basis variables
Previous studies have not evaluated female sex and lower BMI as possible factors associated with postoperative spondylolisthesis. Rosenberg et al. [30] reported that females have a more stable lumbosacral joint, which potentially results in abnormal stress at the intervertebral joints above this level. This could lead to degeneration of the intervertebral disc and ligaments, resulting in hypermobility and spondylolisthesis [30]. While decompression surgery may further increase instability of the lumbar spine when removing (part of) the lamina, facet joints and ligaments, it might be expected that females would have a higher chance of develo** postoperative symptomatic spondylolisthesis, for the same reasons that they have a greater chance of develo** spondylolisthesis itself. Furthermore, females generally have greater longitudinal age-related decrease in the bone mineral density (BMD) than males [31], partly hormonally mediated but possibly also due to their lower body weight. Body mass is positively correlated with BMD, likely because increased mechanical strain on the bones results in increased bone mass to accommodate the greater load [32, 33]. Similarly, lower BMI is associated with a lower BMD. Lower BMD has been shown to increase the risk of pars interarticularis fractures and spondylolisthesis after decompression surgery, due to the inability to manage increased shear forces [10, 11]. Possibly, such a mechanism could underlie the association observed in the present study between lower BMI and postoperative symptomatic spondylolisthesis after decompression surgery.
Univariate regression analysis showed an increased PI and PT in patients with postoperative symptomatic spondylolisthesis, which is comparable with other findings in the literature [34]. Sagittal balance parameters are correlated with one another, and an increased PI and PT are associated with an increased LL. LL also showed a trend for an association with postoperative spondylolisthesis in the univariable regression analysis (P = 0.06). An increased PI and PT, and therefore, an increased LL could result in increased shear forces which could lead to increased stress of pars interacticularis as mentioned above [34].
Surgical level-basis variables
Previous studies mostly described disc height, facet joint angles, facet joint degeneration and preoperative spondylolisthesis as possible associated factors for postoperative spondylolisthesis [2, 4, 5, 9,10,11]. Ramhmdani et al. and Blumenthal et al. found significantly increased disc height in patients with postoperative spondylolisthesis, which fits with the notion of disc height collapse being one of the re-stabilization mechanisms to minimize intervertebral motion [2, 5, 35]. Blumenthal et al. [5] reported that a disc height greater than 6.5 mm was a possible risk factor; however, they only included patients with preoperative degenerative spondylolisthesis, which is different from the present study. Ramhmdani et al. [2] did not perform a regression analysis and had a prevalence of only 10 patients with postoperative spondylolisthesis.
In their multivariable regression analysis, Blumenthal et al. also found that more sagittal-oriented facet angles were a risk factor for postoperative spondylolisthesis [5], similar to our findings.
Sato et al. described an increased risk of same-segment disease, including postoperative spondylolisthesis, after decompression surgery in patients with degenerative spondylolisthesis, when facet joint degeneration was more severe [9]. This supports the findings of our study, where facet joint degeneration grade 2 was associated with lower probability of postoperative symptomatic spondylolisthesis than grade 3, in univariable regression analysis. Supporting the findings of the present study, preoperative spondylolisthesis has previously been described as a risk factor for postoperative spondylolisthesis after decompression surgery. Decompression surgery at levels with preoperative spondylolisthesis appears to increase instability by altering the segment biomechanics even more [4, 5, 9].
Level L4L5 is the most common level to develop degenerative spondylolisthesis. Ilio-lumbar ligaments keep L5 strongly in an anatomical position, mostly by its posterior bands [36, 37]. This stabilizes the L5 vertebrae and may increase the biomechanical forces upon L4. In the case of decompression surgery at L4L5, the subsequent biomechanical and iatrogenic changes could lead to earlier decay of this segment [38]. Previous studies have not evaluated greyscale of MF as possible risk factor associated with postoperative spondylolisthesis. Higher greyscale values indicate more intramuscular fat and connective tissue and less muscle tissue. We believe that higher greyscale values could be associated with spondylolisthesis of the spine due to decreased strength of the muscle and hence less active protection against instability. However, the confidence interval resulting from the univariable regression analysis was large (95%CI 1.845–63.836), which could be explained by the large standard deviation of this ratio (SD 0.1, range 0.02–1.48) due to interpatient variability.
Limitations and future recommendations
To our knowledge, this retrospective study includes the largest number of patients evaluated to date to determine factors associated with postoperative symptomatic spondylolisthesis. However, the study has various weaknesses that need to be highlighted. The retrospective nature of the study increases its risk of bias. Patients were excluded if they had incomplete data, which was the case for a total of 138 patients (16% of all eligible patients). It is not known whether this group would have altered the results; these patients could have gone to other clinics in the case of complaints or indeed had no recurrent symptoms. Another risk of bias is that postoperative imaging was only performed for a select group of patients. In both clinics, patients only received postoperative MRI scans in the case of (novel) neurological complaints, not if they were asymptomatic. Some patients could have been symptomatic, but chosen not to come in for a consultation and/or imaging that would have allowed for postoperative spondylolisthesis to be diagnosed. Only symptomatic postoperative spondylolisthesis was considered an event. We believed that only patients who developed novel complaints after decompression surgery due to spondylolisthesis were relevant, since it is probably this very scenario (i.e. higher risk of postoperative symptomatic spondylolisthesis and the possible need for reoperation) for which an exploration of possible associations would be most useful. Furthermore, progression of the postoperative spondylolisthesis in patients with preoperative spondylolisthesis, could be part of the natural history of the spondylolisthesis of the patient. Another risk of bias concerned the measurement of spondylolisthesis on MRI scans instead of CRs in the upright standing position. MRI scans could have been falsely negative for spondylolisthesis due to realignment in the supine position [39]. A previous study showed that in 5.1% of patients with a Meyerding grade I or II on CR, no slippage was seen on MRI scans [40]. Additionally, postoperative spondylolisthesis was measured by two researchers, but there was no minimum threshold for spondylolisthesis that had to be met. This could have led to the inclusion of patients in the postoperative symptomatic spondylolisthesis group based on measurement errors made by both researchers.
If a routine follow-up of 2 years was not available, patients were actively approached. This period could, however, be too short, given that it has been reported that most patients develop postoperative symptomatic spondylolisthesis within three years after decompression surgery [2, 3, 9], albeit with recurrent symptoms being present after a mean of 19.0 months [2].
CSA of paravertebral muscles were determined in the same plane as the intervertebral disc. Especially in the case of hyperlordotic vertebral levels, it is possible that there could be error introduced by the CSA not being measured truly cross-sectionally.
Based on this study, recommendations for future prospective research can be made. First, previous studies have identified BMD as possible risk factor for postoperative spondylolisthesis [10, 11]. This variable was not included in the present study, because of its retrospective nature and the lack of available baseline information on this particular variable. Second, CRs of the lumbar spine were only available for 488 patients, resulting in less data on sagittal alignment. Furthermore, future studies could use CRs as the diagnostic tool to determine pre- and postoperative spondylolisthesis in the standing position, to obviate the case where MRI scans are perhaps negative for spondylolisthesis due to being taken in the supine position [39]. Third, in this study, facet joint effusion was recorded as a binary variable with “yes” or “no.” Future research should measure effusion as a continuous variable, given that a larger amount could result in a higher risk of spondylolisthesis compared with no or a small amount of effusion [41].
Fourth, this explorative study highlighted factors associated with postoperative symptomatic spondylolisthesis after lumbar decompression surgery. The results could be explored in future research when devising patient selection or stratification criteria or performing subgroup analyses in studies comparing, e.g. decompression alone versus additional fusion, with respect to revision or patient reported outcome measures. Pearson et al. of the SPORT-group [42] determined which factors led to a greater benefit from surgery (laminectomy with and without fusion) over non-operative care in patients with degenerative spondylolisthesis. Besides location of stenosis and severity of stenosis, they did not include other morphological variables in their analyses, which could be important to use in future research and large multivariate models.
Conclusion
In this explorative retrospective study, independent factors in relation to patient characteristics associated with postoperative symptomatic spondylolisthesis were identified and included female sex. Higher BMI was associated with lower probability of postoperative symptomatic spondylolisthesis. When determining associations in relation to the operated vertebral level(s), preoperative spondylolisthesis, more sagittal orientated facet angles and level of surgery were associated with postoperative symptomatic spondylolisthesis. These associations could be used as basis for devising selection criteria for patients, to stratify patients, or to perform subgroups analyses in future studies regarding decompression surgery or decompression versus additional fusion.
References
Zaina F et al. (2016) Surgical versus nonsurgical treatment for lumbar spinal stenosis. Spine 41(14):E857–E868
Ramhmdani S et al. (2018) Iatrogenic spondylolisthesis following open lumbar laminectomy: case series and review of the literature. World Neurosurg 113:e383–e390
Ahmad S et al. (2017) The outcome of decompression alone for lumbar spinal stenosis with degenerative spondylolisthesis. Eur Spine J 26:414–419
Guha D, Heary RF, Shamji MF (2015) Iatrogenic spondylolisthesis following laminectomy for degenerative lumbar stenosis: systematic review and current concepts. Neurosurg Focus 39(4):E9
Blumenthal C et al. (2013) Radiographic predictors of delayed instability following decompression without fusion for degenerative grade I lumbar spondylolisthesis. J Neurosurg Spine 18:340–346
Försth P et al. (2016) A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med 374(15):1413–1423
Ghogawala Z et al. (2016) Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med 374(15):1424–1434
Austevoll IM et al. (2021) Decompression with or without fusion in degenerative lumbar spondylolisthesis. N Engl J Med 385:526–538
Sato S et al. (2015) Reoperation rate and risk factors of elective spinal surgery for degenerative spondylolisthesis: minimum 5-year follow-up. Spine J 15:1536–1544
Bisschop A et al. (2012) The impact of bone mineral density and disc degeneration on shear strength and stiffness of the lumbar spine following laminectomy. Eur Spine J 21:530–536
Bisschop A et al. (2012) Which factors prognosticate spinal instability following lumbar laminectomy? Eur Spine J 21:2640–2648
Thakar S et al. (2016) Lumbar paraspinal muscle morphometry and its correlations with demographic and radiological factors in adult isthmic spondylolisthesis: a retrospective review of 120 surgically managed cases. J Neurosurg Spine 24:679–685
Liu J et al. (2016) A comparative study of perioperative complications between transforaminal versus posterior lumbar interbody fusion in degenerative lumbar spondylolisthesis. Eur Spine J 25:1575–1580
Zhang F et al. (2018) Correlation between lumbar intervertebral disc height and lumbar spine sagittal alignment among asymptomatic Asian young adults. J Orthop Surg Res 13(34):1–7
Pfirrmann CWA et al. (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26(17):1873–1878
Xu WB et al. (2016) Facet orientation and tropism: associations with asymmetric lumbar paraspinal and psoas muscle parameters in patients with chronic low back pain. J Back Musculoskelet Rehabil 29:581–586
Gao T et al. (2017) Correlation between facet tropism and lumbar degenerative disease: a retrospective analysis. BMC Musculoskelet Disord 18(483):1–7
Liu X et al. (2018) Facet sagittal orientation: possible role in the pathology of degenerative lumbar spinal stenosis. Spine 43(14):955–958
Shinto K et al. (2019) Prevalence of facet effusion and its relationship with lumbar spondylolisthesis and low back pain: the Wakayama spine study. J Pain Res 12:3521–3528
Weishaupt D et al. (1999) MR imaging and CT in osteoarthritis of the lumbar facet joints. Skeletal Radiol 28:215–219
D’hooge R et al. (2012) Increased intramuscular fatty infiltration without differences in lumbar muscle cross-sectional area during remission of unilateral low back pain. Man Ther 17:584–588
Crawford RJ et al. (2017) Manually defining regions of interest when quantifying paravertebral muscles fatty infiltration from axial magnetic resonance imaging: a proposed method for the lumbar spine with anatomical crossreference. BMC Musculoskelet Disord 18(25):1–11
Sugawara K, Katayose M, Watanabe K (2016) The variation in the lumbar facet joint orientation in an adult asian population and its relationship with the cross-sectional area of the multifidus and erector spinae. Asian Spine J 10(5):886–892
Lee JC et al. (2008) Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis; comparison with the normal controls. Spine 33(3):318–325
Valentina S, Licka T, Elliott J (2015) Age and side-related morphometric MRI evaluation of trunk muscles in people without back pain. Man Ther 20(1):90–95
Ntilikina Y et al. (2017) Open versus percutaneous instrumentation in thoracolumbar fractures: magnetic resonance imaging comparison of paravertebral muscles after implant removal. J Neurosurg Spine 27:235–241
Labelle H et al. (2004) Spondylolisthesis, pelvic incidence, and spinopelvic balance: a correlation study. Spine 29(18):2049–2054
Le Huec JC et al. (2011) Pelvic parameters: origin and significance. Eur Spine J 20(5):S564–S571
Peduzzi P et al. (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49(12):1373–1379
Rosenberg NJ (1975) Degenerative spondylolisthesis. Predisposing factors. J Bone Jt Surg Am 57(4):467–474
Warming L, Hassager C, Christiansen C (2002) Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int 13:105–112
Bainbridge KE et al. (2004) Risk factors for low bone mineral density and the 6-year rate of bone loss among premenopausal and perimenopausal women. Osteoporos Int 15:439–446
Rubin CT, Lanyon LE (1985) Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int 37:411–417
Tebet MA (2014) Current concepts on the sagittal balance and classification of spondylolysis and spondylolisthesis. Rev Bras Ortop 49(1):3–12
Ramakrishna VAS et al. (2018) Mild (not severe) disc degeneration is implicated in the progression of bilateral L5 spondylolysis to spondylolisthesis. BMC Musculoskelet Disord 19(98):1–11
Leong JCY et al. (1986) The biomechanical functions of the iliolumbar ligament in maintaining stability of the lumbosacral junction. Spine 12(7):669–674
Aihara T et al. (2000) Biomechanical functions of the iliolumbar ligament in L5 spondylolysis. J Orthop Sci 5(3):238–242
DeVine JG, Schenk-Kisser JM, Skelly AC (2012) Risk factors for degenerative spondylolisthesis: a systematic review. Evid Based Spine Care J 3(2):25–34
Viswanathan VK et al. (2018) Comparative utility of dynamic and static imaging in the management of lumbar spondylolisthesis. World Neurosurg 117:e507–e513
Alvi MA et al. (2019) Assessing the differences in measurement of degree of spondylolisthesis between supine MRI and erect X-ray: an institutional analysis of 255 cases. Oper Neurosurg 18(4):438–443
Caterini R et al. (2011) The correlation between exaggerated fluid in lumbar facet joints and degenerative spondylolisthesis: prospective study of 52 patients. J Orthopaed Traumatol 12:87–91
Pearson AM et al. (2013) Who should have surgery for degenerative spondylolisthesis?: Treatment effect predictors in SPORT. Spine (Phila Pa 1976) 1(38):1–23
Acknowledgements
We would like to thank Dr. A. Merry for the assistance in optimizing the statistical analyses of the data. We would like to thank D. O’Riordan for the arrangements for data collection in Schulthess Klinik, Zurich, Switzerland. We would like to thank the following researchers for their contribution to the data collection: J. Gulikers, A. Wall, F. Freriks, K. van Leeuwen, R. Droeghaag, G. Balaban, K. Clift, S. Dosch, A. Fröhlich and S. Nauer.
Funding
Travel funding for Coordinating Investigator (IC) by DePuy. Travel Grant for Coordinating Investigator (IC) by ZonMW.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Measurements of morphological variables
Measurement of facet joint angle. Two lines were placed between two peaks (red arrow) of superior articular facet joints towards the centre of the vertebral body and another line midsagittal through the processes spinosus. The angle between the lines was the facet joint angle. Facet joint tropism was present if the difference between left and right facet joint angle was above 10°
Measurement of muscle CSA and ROI of m. psoas, m. multifidus and m. erector spinae on the right side. The relative CSA of the muscles was calculated by dividing muscle CSA by vertebral body CSA. The relative ROI of the muscles was calculated by dividing muscle ROI by fat tissue ROI. The mean of the left and right relative CSA and ROI of the muscles was calculated by dividing the sum by two
Appendix 2: Flow chart

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Caelers, I.J.M.H., Mannion, A.F., Haschtmann, D. et al. Factors associated with an increased risk of develo** postoperative symptomatic lumbar spondylolisthesis after decompression surgery: an explorative two-centre international cohort study. Eur Spine J 32, 462–474 (2023). https://doi.org/10.1007/s00586-022-07403-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-022-07403-8