Abstract
Purpose
Lipegfilgrastim (Lonquex, Teva Pharma B.V.) is approved for reduction in neutropenia duration and febrile neutropenia incidence. In the framework of lipegfilgrastim regulatory approval in the EU, the Health Authorities requested a drug utilization study. This study was conducted to characterize prescribing patterns of lipegfilgrastim and quantify the extent of on- and off-label use of lipegfilgrastim in real-world setting in Europe.
Methods
Information on lipegfilgrastim use between January 2014 and March 2020 was abstracted from medical records in hospital and outpatient clinical settings. Indication for lipegfilgrastim was classified either as on-label or off-label use according to pre-determined criteria. The primary endpoint was the extent of lipegfilgrastim off-label use based on the most recent lipegfilgrastim cycle.
Results
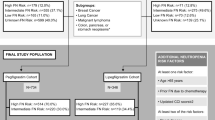
Records of 481 patients were obtained from five European countries. Lipegfilgrastim was most commonly prescribed for prevention of neutropenia by oncologists and hematologists. Patients who were administered lipegfilgrastim were primarily ≥ 55 years old (65.1%) and female (65.7%). The most frequent underlying diagnosis was breast cancer (38.3%).
For the most recent lipegfilgrastim cycle, on-label use was recorded in 452/459 patients with no missing data (98.5%), while off-label use was recorded in 7/459 patients (1.5%). The majority of off-label use was attributed to use with non-cytotoxic chemotherapy (57.1%). Off-label use of lipegfilgrastim across all treatment cycles with no missing data was 11/1547 cycles (0.7%).
Conclusion
Using real-world data, these findings confirm the low rate of lipegfilgrastim off-label use as reported in a preceding feasibility study, indicating very high adherence to the approved indication.



Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article/supplementary material.
Code availability
Not applicable.
References
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47(1):8–32. https://doi.org/10.1016/j.ejca.2010.10.013
Kouroukis CT, Chia S, Verma S, Robson D, Desbiens C, Cripps C, Mikhael J (2008) Canadian supportive care recommendations for the management of neutropenia in patients with cancer. Curr Oncol 15(1):9–23. https://doi.org/10.3747/co.2008.198
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24(19):3187–3205. https://doi.org/10.1200/jco.2006.06.4451
Crawford J, Caserta C, Roila F (2010) Hematopoietic growth factors: ESMO clinical practice guidelines for the applications. Ann Oncol 21(Suppl 5):v248-251. https://doi.org/10.1093/annonc/mdq195
Klastersky J, de Naurois J, Rolston K, Rapoport B, Maschmeyer G, Aapro M, Herrstedt J (2016) Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann Oncol 27(suppl 5):v111–v118. https://doi.org/10.1093/annonc/mdw325
Lucas AJ, Olin JL, Coleman MD (2018) Management and preventive measures for febrile neutropenia. P T 43(4):228–232
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, Goldberg JM, Khatcheressian JL, Leighl NB, Perkins CL, Somlo G, Wade JL, Wozniak AJ, Armitage JO (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33(28):3199–3212. https://doi.org/10.1200/jco.2015.62.3488
Amgen Ltd, UK Neupogen (filgrastim) Summary of Product Characteristics. https://www.medicines.org.uk/emc/product/608/smpc#INDICATIONS. Accessed 2 April 2021
Bruns I, Steidl U, Kronenwett R, Fenk R, Graef T, Rohr UP, Neumann F, Fischer J, Scheid C, Hübel K, Haas R, Kobbe G (2006) A single dose of 6 or 12 mg of pegfilgrastim for peripheral blood progenitor cell mobilization results in similar yields of CD34+ progenitors in patients with multiple myeloma. Transfusion (Paris) 46(2):180–185. https://doi.org/10.1111/j.1537-2995.2006.00699.x
Fruehauf S, Klaus J, Huesing J, Veldwijk MR, Buss EC, Topaly J, Seeger T, Zeller LW, Moehler T, Ho AD, Goldschmidt H (2007) Efficient mobilization of peripheral blood stem cells following CAD chemotherapy and a single dose of pegylated G-CSF in patients with multiple myeloma. Bone Marrow Transplant 39(12):743–750. https://doi.org/10.1038/sj.bmt.1705675
Kroschinsky F, Hölig K, Platzbecker U, Poppe-Thiede K, Ordemann R, Blechschmidt M, Oelschlaegel U, Schaich M, Hänel M, Bornhäuser M, Ehninger G (2006) Efficacy of single-dose pegfilgrastim after chemotherapy for the mobilization of autologous peripheral blood stem cells in patients with malignant lymphoma or multiple myeloma. Transfusion (Paris) 46(8):1417–1423. https://doi.org/10.1111/j.1537-2995.2006.00911.x
Teva Pharma BV, UK Lonquex (lipegfilgrastim) Summary of Product Characteristics. https://www.medicines.org.uk/emc/product/5422/smpc#INDICATIONS. Accessed 2 April 2021
Bondarenko I, Gladkov OA, Elsaesser R, Buchner A, Bias P (2013) Efficacy and safety of lipegfilgrastim versus pegfilgrastim: a randomized, multicenter, active-control phase 3 trial in patients with breast cancer receiving doxorubicin/docetaxel chemotherapy. BMC Cancer 13:386. https://doi.org/10.1186/1471-2407-13-386
Gladkov OA, Buchner A, Bias P, Müller U, Elsässer R (2016) Chemotherapy-associated treatment burden in breast cancer patients receiving lipegfilgrastim or pegfilgrastim: secondary efficacy data from a phase III study. Support Care Cancer 24(1):395–400. https://doi.org/10.1007/s00520-015-2803-9
Link H, Illerhaus G, Martens UM, Salar A, Depenbusch R, Köhler A, Engelhardt M, Mahlmann S, Zaiss M, Lammerich A, Bias P, Buchner A (2021) Efficacy and safety of lipegfilgrastim versus pegfilgrastim in elderly patients with aggressive B cell non-Hodgkin lymphoma (B-NHL): results of the randomized, open-label, non-inferiority AVOID neutropenia study. Support Care Cancer 29(5):2519–2527. https://doi.org/10.1007/s00520-020-05711-7
Volovat C, Bondarenko IM, Gladkov OA, Elsässer R, Buchner A, Bias P, Müller U (2015) Phase III, randomized, double-blind, placebo-controlled, multicenter study of lipegfilgrastim in patients with non-small cell lung cancer receiving myelosuppressive therapy. Springer Plus 4:316. https://doi.org/10.1186/s40064-015-1067-7
Bond TC, Szabo E, Gabriel S, Klastersky J, Tomey O, Mueller U, Schwartzberg L, Tang B (2018) Meta-analysis and indirect treatment comparison of lipegfilgrastim with pegfilgrastim and filgrastim for the reduction of chemotherapy-induced neutropenia-related events. J Oncol Pharm Pract 24(6):412–423. https://doi.org/10.1177/1078155217714859
Bondarenko IM, Bias P, Buchner A (2016) Incidence of bone pain in patients with breast cancer treated with lipegfilgrastim or pegfilgrastim: an integrated analysis from phase II and III studies. Support Care Cancer 24(1):267–273. https://doi.org/10.1007/s00520-015-2777-7
Fietz T, Lück A, Schulz H, Harde J, Losem C, Grebhardt S, Wolff T, Potthoff K, Müller U, Zaiss M, Kurbacher CM (2019) Prophylaxis of chemotherapy-induced neutropenia and febrile neutropenia with lipegfilgrastim in 2489 cancer patients: final results from the non-interventional study NADIR. Curr Med Res Opin 35(7):1127–1138. https://doi.org/10.1080/03007995.2018.1560200
Fontaine C, Claes N, Graas MP, Samani KK, Vuylsteke P, Vulsteke C (2021) Effect of lipegfilgrastim administration as prophylaxis of chemotherapy-induced neutropenia on dose modification and incidence of neutropenic events: real-world evidence from a non-interventional study in Belgium and Luxembourg. Acta Clin Belg 76(1):10–15. https://doi.org/10.1080/17843286.2019.1646539
Gessner C, Potthoff K, Frost N (2021) Efficacy and safety of lipegfilgrastim in lung cancer patients receiving myelosuppressive chemotherapy in a real-world setting: results of an analysis of pooled data from two non-interventional european studies. Oncol Res Treat 44(3):93–102. https://doi.org/10.1159/000512594
Holubec L, Polivka J, Lisnerova L, Kubikova T, Safanda M (2017) The effectiveness of febrile neutropenia prophylaxis with lipegfilgrastim in routine clinical practice. In Vivo 31(3):303–306. https://doi.org/10.21873/invivo.11059
Merseburger AS, Geiges G, Klier J, Wiesholzer M, Pichler P (2021) Pooled analysis on the effectiveness and safety of lipegfilgrastim in patients with urological malignancies in the real-world setting. Front Oncol 11:655355. https://doi.org/10.3389/fonc.2021.655355
Cortes de Miguel S, Calleja-Hernandez MA, Menjon-Beltran S, Vallejo-Rodriguez I (2015) Granulocyte colony-stimulating factors as prophylaxis against febrile neutropenia. Support Care Cancer 23(2):547–559. https://doi.org/10.1007/s00520-014-2459-x
Panopoulos AD, Watowich SS (2008) Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 42(3):277–288. https://doi.org/10.1016/j.cyto.2008.03.002
Saloustros E, Tryfonidis K, Georgoulias V (2011) Prophylactic and therapeutic strategies in chemotherapy-induced neutropenia. Expert Opin Pharmacother 12(6):851–863. https://doi.org/10.1517/14656566.2011.541155
Silvestris N, Del Re M, Azzariti A, Maiello E, Lombardi L, Cinieri S, Guarini A, Brunetti AE, Delcuratolo S, De Vita F, Pisconti S, Danesi R, Colucci G (2012) Optimized granulocyte colony-stimulating factor prophylaxis in adult cancer patients: from biological principles to clinical guidelines. Expert Opin Ther Targets 16(Suppl 2):S111-117. https://doi.org/10.1517/14728222.2011.652089
European Medicines Agency (EMA). Committee for medicinal products for human use (CHMP) assessment report: Lonquex. 30 May 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002556/WC500148382.pdf. Accessed 18 July 2018
Kaplan S, Lang N, Gasparic M, Rainville C, Haralabopoulos G, Borroni E (2019) Prescribing patterns from medical chart abstraction of patients administered lipegfilgrastim: a pilot study in Europe. J Drug Assess 8(1):70–76. https://doi.org/10.1080/21556660.2019.1604376
Tralongo AC, Antonuzzo A, Pronzato P, Sbrana A, Turrini M, Zoratto F, Danova M (2020) Management of chemotherapy-induced neutropenia in patients with cancer: 2019 guidelines of the Italian Medical Oncology Association (AIOM). Tumori 106(4):273–280. https://doi.org/10.1177/0300891620927093
Becker PS, Griffiths EA, Alwan LM, Bachiashvili K, Brown A, Cool R, Curtin P, Dinner S, Gojo I, Hicks A, Kallam A, Kidwai WZ, Kloth DD, Kraut EH, Landsburg D, Lyman GH, Miller R, Mukherjee S, Patel S, Perez LE, Poust A, Rampal R, Rosovsky R, Roy V, Rugo HS, Shayani S, Vasu S, Wadleigh M, Westbrook K, Westervelt P, Burns J, Keller J, Pluchino LA (2020) NCCN guidelines insights: hematopoietic growth factors, Version 1.2020. J Natl Compr Canc Netw 18(1):12–22. https://doi.org/10.6004/jnccn.2020.0002
Burris HA, Belani CP, Kaufman PA, Gordon AN, Schwartzberg LS, Paroly WS, Shahin S, Dreiling L, Saven A (2010) Pegfilgrastim on the same day versus next day of chemotherapy in patients with breast cancer, non-small-cell lung cancer, ovarian cancer, and non-hodgkin’s lymphoma: results of four multicenter, double-blind, randomized phase II studies. J Oncol Pract 6(3):133–140. https://doi.org/10.1200/jop.091094
Skarlos DV, Timotheadou E, Galani E, Samantas E, Grimani I, Lianos E, Aravantinos G, Xanthakis I, Pentheroudakis G, Pectasides D, Fountzilas G (2009) Pegfilgrastim administered on the same day with dose-dense adjuvant chemotherapy for breast cancer is associated with a higher incidence of febrile neutropenia as compared to conventional growth factor support: matched case-control study of the Hellenic Cooperative Oncology Group. Oncology 77(2):107–112. https://doi.org/10.1159/000229504
Crawford J, Becker PS, Armitage JO, Blayney DW, Chavez J, Curtin P, Dinner S, Fynan T, Gojo I, Griffiths EA, Hough S, Kloth DD, Kuter DJ, Lyman GH, Mably M, Mukherjee S, Patel S, Perez LE, Poust A, Rampal R, Roy V, Rugo HS, Saad AA, Schwartzberg LS, Shayani S, Talbott M, Vadhan-Raj S, Vasu S, Wadleigh M, Westervelt P, Burns JL, Pluchino L (2017) Myeloid growth factors, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 15(12):1520–1541. https://doi.org/10.6004/jnccn.2017.0175
Gawade PL, Li S, Henry D, Smith N, Belani R, Kelsh MA, Bradbury BD (2020) Patterns of granulocyte colony-stimulating factor prophylaxis in patients with cancer receiving myelosuppressive chemotherapy. Support Care Cancer 28(9):4413–4424. https://doi.org/10.1007/s00520-020-05295-2
Conti RM, Bernstein AC, Villaflor VM, Schilsky RL, Rosenthal MB, Bach PB (2013) Prevalence of off-label use and spending in 2010 among patent-protected chemotherapies in a population-based cohort of medical oncologists. J Clin Oncol 31(9):1134–1139. https://doi.org/10.1200/jco.2012.42.7252
Eaton AA, Sima CS, Panageas KS (2016) Prevalence and safety of off-label use of chemotherapeutic agents in older patients with breast cancer: estimates from SEER-medicare data. J Natl Compr Canc Netw 14(1):57–65
Morris J (2012) The use of observational health-care data to identify and report on off-label use of biopharmaceutical products. Clin Pharmacol Ther 91(5):937–942. https://doi.org/10.1038/clpt.2012.30
European Commission, Directorate-General for Health, Food Safety, Marjolein W, Lisman J, Hoebert J, Moltó Puigmarti C, Dijk L, Langedijk J, Marchange S, Damen N, Vervloet M (2019) Study on off-label use of medicinal products in the European Union: report. Publications Office.
Bocquet F, Paubel P, Fusier I, Cordonnier AL, Le Pen C, Sinègre M (2014) Biosimilar granulocyte colony-stimulating factor uptakes in the EU-5 markets: a descriptive analysis. Appl Health Econ Health Policy 12(3):315–326. https://doi.org/10.1007/s40258-014-0087-8
Nordic MDS Group. Guidelines for the diagnosis and treatment of myelodysplastic syndrome and chronic myelomonocytic leukemia, 8th update, May 2017. https://www.nmds.org/attachments/article/92/Guidelines%20for%20the%20diagnosis%20and%20treatment%20of%20MDS%20and%20CMML_17.pdf. Accessed April 2 2021
Barnes G, Pathak A, Schwartzberg L (2014) G-CSF utilization rate and prescribing patterns in United States: associations between physician and patient factors and GCSF use. Cancer Med 3(6):1477–1484. https://doi.org/10.1002/cam4.344
Hawkins A, Murphy A, McNamara M, Gawade PL, Belani R, Kelsh MA (2020) A survey of oncologists’ perceptions and opinions regarding the use of granulocyte colony-stimulating factors. J Cancer Educ 35(1):178–186. https://doi.org/10.1007/s13187-019-01638-8
Acknowledgements
The authors would like to acknowledge George Haralabopoulos, PhD, from PRA for executing the study.
Funding
This study was funded by Teva Pharmaceutical Industries Ltd.
Author information
Authors and Affiliations
Contributions
SK: study concept and design, interpretation of the data, manuscript writing. DIB: interpretation of the data. CR: project administration, acquisition of data, and interpretation of the data. NG: Statistical analysis and interpretation of the data. All the authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval and a waiver for patient informed consent were obtained for all participating centers. The study was performed in accordance with local ethics committees and regulatory requirements in participating countries.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
All authors are employees of Teva Pharmaceutical Industries Ltd (and/or its affiliates). The sponsor had a role in study design, analysis and interpretation of data, and manuscript writing.
Role of the sponsor
The sponsor had a role in the design and conduct of the study; study supervision, management, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect those of Teva Pharmaceutical Industries Ltd and/or its affiliates.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaplan, S., Bogojevic, D.I., Rainville, C. et al. A multinational, drug utilization study of lipegfilgrastim use in real-world setting in Europe. Support Care Cancer 30, 9191–9201 (2022). https://doi.org/10.1007/s00520-022-07341-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07341-7