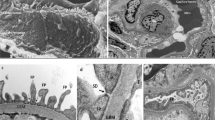
Abstract
Nephrotic syndrome (NS) in infancy includes NS of Finnish type (mutation of the nephrin gene), diffuse mesangial sclerosis (idiopathic or linked to WT1 mutation), idiopathic NS, most often steroid resistant, and NS related to infections during pregnancy (virus, syphilis, toxoplasmosis). Later in life, NS has a large variety of etiologies. It has been described in association with neuromuscular symptoms, deafness, and diabetes in a few children and adults with respiratory chain (RC) disorders. To date, however, NS has never been observed in neonates with RC disorders. Here, we report RC deficiency in one infant with certain congenital NS and two siblings with acute neonatal cardiac and renal disease with probable NS. Although clinical and histopathological presentations were initially close to congenital NS of Finnish type, clinical outcome was atypical and nephrin mutation was excluded. Mitochondrial RC complex II+V deficiency was identified in the three patients. Based on these observations, we suggest that RC disorders should be considered in patients with congenital NS.



Similar content being viewed by others
References
Habib R (1993) Nephrotic syndrome in the first year of life. Pediatr Nephrol 7:347–353
Salomon R, Gubler MC, Niaudet P (2000) Genetics of the nephrotic syndrome. Curr Opin Pediatr 12:129–134
Khoshnoodi J, Tryggvason K (2001) Congenital nephrotic syndromes. Curr Opin Genet Dev 11:322–327
Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K (1998) Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1:575–582
Habib R, Loirat C, Gubler MC, Niaudet P, Bensman A, Levy M, Broyer M (1985) The nephropathy associated with male pseudohermaphrodism and Wilm’s tumor (Drash syndrome): a distinctive glomerular lesion—report of 10 cases. Clin Nephrol 24:269–278
Boute N, Gribouval O, Roselli S, Benessy S, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C (2000) NPHS2 encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24:349–354
Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR (2000) Mutations in ACTN4, encoding actinin-4 cause familial focal segmental glomerulosclerosis. Nat Genet 24:251–256
Winn MP, Conlon PJ, Lynn KL, Howell DN, Slotterbeck BD, Smith AH, Graham FL, Bembe M, Quarles LD, Pericak-Vance MA, Vance JM (1999) Linkage of a gene causing familial focal segmental glomerulosclerosis to chromosome 11 and further evidence of genetic heterogeneity. Genomics 58:113–120
Brun P, Ogier de Baulny H, Peuchmaur M, Lombes A, Simon D, Loirat C (1994) Les atteintes rénales des cytopathies mitochondriales. In: Arthuis M, Beaufils F, Caille B, Dommergues JP, Fontaine JL, Griscelli C, Job JC, Lasfargues G, Lenoir G, Mathieu H, Paillerets F de, Saudubray JM (eds) Journées Parisiennes de Pédiatrie Flammarion Médecine Sciences, Paris, pp 227–234
Nakamura S, Yoshinari M, Doi Y, Yoshizumi H, Katafuchi R, Yokomizo Y, Nishiyama K, Wakisaka M, Fujishima M (1999) Renal complication in patients with diabetes mellitus associated with an A to G mutation of mitochondrial DNA at the 3243 position of leucine tRNA. Diabetes Res Clin Pract 44:183–189
Tanaka K, Ueno M, Atsumi T, Fukagawa M, Koike Y (1986) A case of mitochondrial encephalomyopathy with nephrotic syndrome. Rinsho Shinkeigaku 26:1190–1196
Hirano M, Konishi K, Arata N, Iyori M, Saruta T, Kuramochi S, Akizuki M (2002) Renal complications in a patient with A to G mutation of mitochondrial DNA at the 3243 position of leucine tRNA. Intern Med 41:113–118
Hameed R, Raafat F, Ramani P, Gray G, Roper HP, Milford DV (2001) Mitochondrial cytopathy presenting with focal segmental glomerulosclerosis, hypoparathyroïdism, sensorineural deafness, and progressive neurological disease. Postgrad Med 77:523–526
Niaudet P, Rötig A (1996) Renal involvement in mitochondrial cytopathies. Pediatr Nephrol 10:368–373
Rotig A, Appelkvist EL, Geromel V, Chretien D, Kadhom N, Edery P, Lebideau M, Dallner G, Munnich A, Ernster L, Rustin P (2000) Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet 356:391–395
Mochizuki H, Joh K, Kawame H, Imadachi A, Nozaki H, Ohashi T, Usui N, Eto Y, Kanetsuna Y, Aizawa S (1996) Mitochondrial encephalomyopathy preceded by de Toni-Debré-Fanconi syndrome or focal segmental glomerulosclerosis. Clin Nephrol 46:347–352
Shigemoto M, Yoshimasa Y, Yamamoto Y, Hayashi T, Suga J, Inoue G, Okamoto M, **gami H, Tsuda K, Yamamoto T, Yagura T, Oishi M, Tsujii S, Kuzuya H, Nakao K (1998) Clinical manifestations due to point mutation of the mitochondrial tRNAleu(UUR) gene in five families with diabetes melitus. Intern Med 37:265–272
Kurogouchi F, Oguchi T, Mawatari E, Yamaura S, Hora K, Takei M, Sekijima Y, Ikeda S, Kiyosawa K (1998) A case of mitochondrial cytopathy with a typical point mutation for MELAS, presenting with severe focal-segmental glomerulosclerosis as main clinical manifestation. Am J Nephrol 18:551–556
Cheong HI, Chae JH, Kim JS, Park HW, Ha IS, Hwang YS, Lee HS, Choi Y (1999) Hereditary glomerulopathy associated with a mitochondrial tRNA(Leu) gene mutation. Pediatr Nephrol 13:477–480
Doleris LM, Hill GS, Chedin P, Nochy D, Bellanne-Chantelot C, Hanslik T, Bedrossian J, Caillat-Zucman S, Cahen-Varsaux J, Bariety J (2000) Focal segmental glomerulosclerosis associated with mitochondrial cytopathy. Kidney Int 58:1851–1858
Hotta O, Inoue CN, Miyabayashi S, Furuta T, Takeuchi A, Taguma Y (2001) Clinical and pathologic features of focal segmental glomerulosclerosis with mitochondrial tRNA(leu(UUR))gene mutation. Kidney Int 59:1236–1243
Rustin P, Chretien D, Bourgeron T, Rötig A, Saudubray JM, Munnich A (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 228:35–51
Munnich A, Rustin P (2001) Clinical spectrum and diagnosis of mitochondrial disorders. Am J Med Genet 106:4–17
Eviatar L, Shanske S, Gauthier B, Abrams C, Maytal J, Slavin M, Valderrama E, DiMauro S (1990) Kearns-Sayre syndrome presenting as renal tubular acidosis. Neurology 40:1761–1763
Szabolcs AMJ, Seije R, Shanske S, Bonilla E, Di Mauro S, D’Agati V (1994) Deletion of mitochondrial DNA: a cause of chronic tubulointerstitial nephropathy. Kidney Int 45:1388–1396
Rotig A, Goutieres F, Niaudet P, Rustin P, Chretien D, Guest G, Mikol J, Gubler MC, Munnich A (1995) Deletion of mitochondrial DNA in patient with chronic tubulointerstitial nephritis. J Pediatr 126:597–601
Niaudet P, Heidet L, Munnich A, Schmitz J, Bouissou F, Gubler MC, Rotig A (1994) Deletion of the mitochondrial DNA in a case of the De Toni-debré-fanconi syndrome and Pearson syndrome. Pediatr Nephrol 8:164–168
Niaudet P, Rötig A (1997) The kidney in mitochondrial cytopathies. Kidney Int 51:1000–1007
Lonlay P de, Valnot I, Barrientos A, Gorbatyuk M, Tzagoloff A, Taanman JW, Benayoun E, Chretien D, Kadhom N, Lombes A, Baulny HO de, Niaudet P, Munnich A, Rustin P, Rotig A (2001) A mutant mitochondrial respiratory chain assembly protein causes complex III deficiency in patients with tubulopathy, encephalopathy and liver failure. Nat Genet 29:57–61
Goto Y, Itami N, Kajii N, Tochimaru H, Endo M, Horai S (1990) Renal tubular involvement mimicking Barter syndrome in a patient with Kearns-Sayre syndrome. J Pediatr 116:904–910
Solin ML, Pitkanen S, Taanman JW, Holthofer H (2000) Mitochondrial dysfunction in congenital nephrotic syndrome. Lab Invest 80:1227–1232
Holthofer H, Kretzler M, Haltia A, Solin ML, Taanman JW, Schagger H, Kriz W, Kerjaschki D, Schlondorff D (1999) Altered gene expression and functions of mitochondria in human nephrotic syndrome. FASEB J 13:523–532
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goldenberg, A., Ngoc, L.H., Thouret, MC. et al. Respiratory chain deficiency presenting as congenital nephrotic syndrome. Pediatr Nephrol 20, 465–469 (2005). https://doi.org/10.1007/s00467-004-1725-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-004-1725-4