Abstract
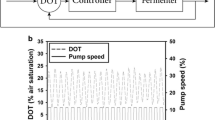
The trisaccharide 1-kestose, a major constituent of commercial fructooligosaccharide (FOS) formulations, shows a superior prebiotic effect compared to higher-chain FOS. The plant sucrose:sucrose 1-fructosyltransferases (1-SST) are extensively used for selective synthesis of lower chain FOS. In this study, enhanced recombinant (r) 1-SST production was achieved in Komagataella phaffii (formerly Pichia pastoris) containing three copies of a codon-optimized Festuca arundinacea 1-SST gene. R1-SST production reached 47 U/mL at the shake-flask level after a 96-h methanol induction phase. A chemostat-based strain characterization methodology was adopted to assess the influence of specific growth rate (µ) on cell-specific r1-SST productivity (Qp) and cell-specific oxygen uptake rate (Qo) under two different feeding strategies across dilution rates from 0.02 to 0.05 h−1. The methanol–sorbitol co-feeding strategy significantly reduced Qo by 46 ± 2.4% compared to methanol-only feeding without compromising r1-SST productivity. Based on the data, a dilution rate of 0.025 h−1 was applied for continuous cultivation of recombinant cells to achieve a sustained r1-SST productivity of 5000 ± 64.4 U/L/h for 15 days.







Similar content being viewed by others
Data availability
The raw data related to this submission will be available on a reasonable request to the corresponding author.
References
Nobre C, Simões LS, Gonçalves DA, Berni P, Teixeira JA (2022) Fructooligosaccharides production and the health benefits of prebiotics. In: Rai AK, Singh SP, Pandey A, Larroche C, Soccol CR (eds) Current developments in biotechnology and bioengineering. Elsevier, pp 109–138
Pengrattanachot N, Thongnak L, Lungkaphin A (2022) The impact of prebiotic fructooligosaccharides on gut dysbiosis and inflammation in obesity and diabetes-related kidney disease. Food Funct 13:5925–5945. https://doi.org/10.1039/D1FO04428A
Dou Y, Yu X, Luo Y, Chen B, Ma D, Zhu J (2022) Effect of fructooligosaccharides supplementation on the gut microbiota in human: a systematic review and meta-analysis. Nutrients 14:3298. https://doi.org/10.3390/nu14163298
Gu J, Cui S, Tang X, Liu Z, Zhao J, Zhang H, Mao B, Chen W (2022) Fructooligosaccharides (FOS) significantly increased the relative abundance of intestinal Bifidobacterium pseudolongum in mice with different genotypes. Curr Res Food Sci 5:2178–2189. https://doi.org/10.1016/j.crfs.2022.10.030
Kaewarsar E, Chaiyasut C, Lailerd N, Makhamrueang N, Peerajan S, Sirilun S (2023) Optimization of mixed inulin, fructooligosaccharides, and galactooligosaccharides as prebiotics for stimulation of probiotics growth and function. Foods 12:1591. https://doi.org/10.3390/foods12081591
Mahalak KK, Firrman J, Narrowe AB, Hu W, Jones SM, Bittinger K, Moustafa AM, Liu L (2023) Fructooligosaccharides (FOS) differentially modify the in vitro gut microbiota in an age-dependent manner. Front Nutr 9:1058910. https://doi.org/10.3389/fnut.2022.1058910
Litvak Y, Byndloss MX, Bäumler AJ (2018) Colonocyte metabolism shapes the gut microbiota. Science. https://doi.org/10.1126/science.aat9076
Costa GT, Vasconcelos QDJS, Abreu GC, Albuquerque AO, Vilar JL, Aragão GF (2021) Systematic review of the ingestion of fructooligosaccharides on the absorption of minerals and trace elements versus control groups. Clin Nutr ESPEN 41:68–76. https://doi.org/10.1016/j.clnesp.2020.11.007
Costa GT, Vasconcelos QD, Aragão GF (2022) Fructooligosaccharides on inflammation, immunomodulation, oxidative stress, and gut immune response: a systematic review. Nutr Rev 80:709–722. https://doi.org/10.1093/nutrit/nuab115
Chen P, Chen F (2023) 1-Kestose, the smallest fructooligosaccharide component, protection for mild to moderate ulcerative colitis patients. Aliment Pharmacol Ther 57:1349–1350. https://doi.org/10.1111/apt.17420
Endo A, Hirano K, Ose R, Maeno S, Tochio T (2020) Impact of kestose supplementation on the healthy adult microbiota in vitro fecal batch cultures. Anaerobe 61:102076. https://doi.org/10.1016/j.anaerobe.2019.102076
Suzuki N, Aiba Y, Takeda H, Fukumori Y, Koga Y (2006) Superiority of 1-kestose, the smallest fructooligosaccharide, to a synthetic mixture of fructooligosaccharides in the selective stimulating activity on bifidobacteria. Biosci Microflora 25:109–116. https://doi.org/10.12938/bifidus.25.109
Tochio T, Kadota Y, Tanaka T, Koga Y (2018) 1-Kestose, the smallest fructooligosaccharide component, which efficiently stimulates Faecalibacterium prausnitzii as well as Bifidobacteria in humans. Foods 7:140. https://doi.org/10.3390/foods7090140
Ni D, Xu W, Zhu Y, Pang X, Lv J, Mu W (2021) Insight into the effects and biotechnological production of kestoses, the smallest fructooligosaccharides. Crit Rev Biotechnol 41:34–46. https://doi.org/10.1080/07388551.2020
Sánchez-Martínez MJ, Soto-Jover S, Antolinos V, Martínez-Hernández GB, López-Gómez A (2020) Manufacturing of short-chain fructooligosaccharides: from laboratory to industrial scale. Food Eng Rev 12:149–172. https://doi.org/10.1007/s12393-020-09209-0
Karkeszová K, Polakovič M (2023) Production of fructooligosaccharides using a commercial heterologously expressed Aspergillus sp, fructosyltransferase. Catalysts 13:843. https://doi.org/10.3390/catal13050843
van Arkel J, Sévenier R, Hakkert JC, Bouwmeester HJ, Koops AJ, van der Meer IM (2013) Tailor-made fructan synthesis in plants: a review. Carbohydr Polym 93:48–56. https://doi.org/10.1016/j.carbpol.2012.02.001
Van den Ende W, Van Laere A (1996) Variation in the in vitro generated fructan pattern from sucrose as a function of the purified chicory root 1-SST and 1-FFT concentrations. J Exp Bot 47:1797–1803. https://doi.org/10.1093/jxb/47.11.1797
Vergauwen R, Van Laere A, Van den Ende W (2003) Properties of fructan:fructan 1-fructosyltransferases from chicory and globe thistle, two Asteracean plants storing greatly different types of inulin. Plant Physiol 133:391–401. https://doi.org/10.1104/pp.103.026807
Ávila-Fernández Á, Olvera-Carranza C, Rudino-Pinera E, Cassab GI, Nieto-Sotelo J, López-Munguía A (2007) Molecular characterization of sucrose: sucrose 1-fructosyltransferase (1-SST) from Agave tequilana Weber var. azul. Plant Sci 173:478–486. https://doi.org/10.1016/j.plantsci.2007.07.009
Vijn I, van Dijken A, Lüscher M, Bos A, Smeets E, Weisbeek P, Wiemken A, Smeekens S (1998) Cloning of sucrose:sucrose 1-fructosyltransferase from onion and synthesis of structurally defined fructan molecules from sucrose. Plant Physiol 117:1507–1513. https://doi.org/10.1104/pp.117.4.1507
De Halleux S, Van Cutsem P (1997) Cloning and sequencing of the 1-SST cDNA from chicory root. Plant Physiol 113:1003–1003
van der Meer IM, Koops AJ, Hakkert JC, van Tunen AJ (1998) Cloning of the fructan biosynthesis pathway of Jerusalem artichoke. Plant J 15:489–500. https://doi.org/10.1046/j.1365-313x.1998.00230.x
Hochstrasser U, Lüscher M, De Virgilio C, Boller T, Wiemken A (1998) Expression of a functional barley sucrose-fructan 6-fructosyltransferase in the methylotrophic yeast Pichia pastoris. FEBS Lett 440:356–360. https://doi.org/10.1016/s0014-5793(98)01487-2
Lüscher M, Hochstrasser U, Vogel G, Aeschbacher R, Galati V, Nelson CJ, Boller T, Wiemken A (2000) Cloning and functional analysis of sucrose:sucrose 1-fructosyltransferase from tall fescue. Plant Physiol 124:1217–1228. https://doi.org/10.1104/pp.124.3.1217
Schroeven L, Lammens W, Van Laere A, Van den Ende W (2008) Transforming wheat vacuolar invertase into a high affinity sucrose:sucrose 1-fructosyltransferase. New Phytol 180:822–831. https://doi.org/10.1111/j.1469-8137.2008.02603.x
Lasseur B, Schroeven L, Lammens W, Le Roy K, Spangenberg G, Manduzio H, Vergauwen R, Lothier J, Prud’homme MP, Van den Ende W (2009) Transforming a fructan:fructan 6G-fructosyltransferase from perennial ryegrass into a sucrose:sucrose 1-fructosyltransferase. Plant Physiol 149:327–339. https://doi.org/10.1104/pp.108.125559
Hernández L, Menéndez C, Pérez ER, Martínez D, Alfonso D, Trujillo LE, Ramírez R, Sobrino A, Mazola Y, Musacchio A, Pimentel E (2018) Fructooligosaccharides production by Schedonorus arundinaceus sucrose: sucrose 1-fructosyltransferase constitutively expressed to high levels in Pichia pastoris. J Biotechnol 266:59–71. https://doi.org/10.1016/j.jbiotec.2017.12.008
Cruz ERP, Garcia LH, García DM, Toledo LET, Rodríguez CM, Legón AS, Ibañez RR, Costa GF, Rodicio JML (2021) U.S. Patent No. 10,982,246. Washington, DC: U.S. Patent and Trademark Office
Pan Y, Yang J, Wu J, Yang L, Fang H (2022) Current advances of Pichia pastoris as cell factories for production of recombinant proteins. Front Microbiol 13:1059777. https://doi.org/10.3389/fmicb.2022.1059777
Curvers S, Brixius P, Klauser T, Thömmes J, Weuster-Botz D, Takors R, Wandrey C (2001) Human chymotrypsinogen B production with Pichia pastoris by integrated development of fermentation and downstream processing. Part 1. Fermentation. Biotechnol Prog 17:495–502. https://doi.org/10.1021/bp000164j
Khatri NK, Hoffman F (2006) Impact of methanol concentration on secreted protein production in oxygen-limited cultures of recombinant Pichia pastoris. Biotechnol Bioeng 93:871–879. https://doi.org/10.1002/bit.20773
Looser V, Bruhlmann B, Bumbak F, Stenger C, Costa M, Camattari A, Fotiadis D, Kovar K (2015) Cultivation strategies to enhance productivity of Pichia pastoris: a review. Biotechnol Adv 33:1177–1193. https://doi.org/10.1016/j.biotechadv.2015.05.008
Schenk J, Balazs K, Jungo C, Urfer J, Wegmann C, Zocchi A, Marison IW, von Stockar U (2008) Influence of specific growth rate on specific productivity and glycosylation of a recombinant avidin produced by a Pichia pastoris Mut + strain. Biotechnol Bioeng 99:368–377. https://doi.org/10.1002/bit.21565
Gautam A, Sahai V, Mishra S (2021) Development of a dual specific growth rate-based fed-batch process for production of recombinant human granulocyte colony-stimulating factor in Pichia pastoris. Bioprocess Biosyst Eng 44:103–112. https://doi.org/10.1007/s00449-020-02427-0
Liu W, **ang H, Zhang T, Pang X, Su J, Liu H, Ma B, Yu L (2020) Development of a new high-cell density fermentation strategy for enhanced production of a fungus β-glucosidase in Pichia pastoris. Front Microbiol 11:1988. https://doi.org/10.3389/fmicb.2020.01988
Gasset A, Garcia-Ortega X, Garrigós-Martínez J, Valero F, Montesinos-Seguí JL (2022) Innovative bioprocess strategies combining physiological control and strain engineering of Pichia pastoris to improve recombinant protein production. Front Bioeng Biotechnol 10:818434. https://doi.org/10.3389/fbioe.2022.818434
Hu R, Cui R, Xu Q, Lan D, Wang Y (2022) Controlling specific growth rate for recombinant protein production by Pichia pastoris under oxidation stress in fed-batch fermentation. Appl Biochem Biotechnol 194:6179–6193. https://doi.org/10.1007/s12010-022-04022-3
Jungo C, Schenk J, Pasquier M, Marison IW, von Stockar U (2007) A quantitative analysis of the benefits of mixed feeds of sorbitol and methanol for the production of recombinant avidin with Pichia pastoris. J Biotechnol 131:57–66. https://doi.org/10.1016/j.jbiotec.2007.05.019
Chen L, Mohsin A, Chu J, Zhuang Y, Liu Y, Guo M (2017) Enhanced protein production by sorbitol co-feeding with methanol in recombinant Pichia pastoris strains. Biotechnol Bioprocess Eng 22:767–773. https://doi.org/10.1007/s12257-017-0011-9
Karbalaei M, Rezaee SA, Farsiani H (2020) Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J Cell Physiol 235:5867–5881. https://doi.org/10.1002/jcp.29583
Nieto-Taype MA, Garcia-Ortega X, Albiol J, Montesinos-Seguí JL, Valero F (2020) Continuous cultivation as a tool toward the rational bioprocess development with Pichia pastoris cell factory. Front Bioeng Biotechnol. 8:632. https://doi.org/10.3389/fbioe.2020.00632
Park J, Liu N, Stephanopoulos G (2023). U.S. Patent No. 11,649,472. Washington, DC: U.S. Patent and Trademark Office
Luengo A, Li Z, Gui DY, Sullivan LB, Zagorulya M, Do BT, Ferreira R, Naamati A, Ali A, Lewis CA, Thomas CJ, Spranger S, Matheson NJ, Vander Heiden MG (2021) Increased demand for NAD+ relative to ATP drives aerobic glycolysis. Mol Cell 81:691-707.e6. https://doi.org/10.1016/j.molcel.2020.12.012
Miles D, Busser JK, Stalder C, Higgins DR (1998) Isolation of nucleic acids. In: Higgins D, Cregg JM (eds) Pichia protocols. Humana Press, New Jersey, Methods in molecular biology, pp 73–80
Maity N, Jaswal AS, Gautam A, Sahai V, Mishra S (2022) High level production of stable human serum albumin in Pichia pastoris and characterization of the recombinant product. Bioprocess Biosyst Eng 45:409–424. https://doi.org/10.1007/s00449-021-02670-z
Goh HY, Sulu M, Alosert H, Lewis GL, Josland GD, Merriman DE (2020) Applications of off-gas mass spectrometry in fed-batch mammalian cell culture. Bioprocess Biosyst Eng 43:483–493. https://doi.org/10.1007/s00449-019-02242-2
Canales C, Altamirano C, Berrios J (2015) Effect of dilution rate and methanol-glycerol mixed feeding on heterologous Rhizopus oryzae lipase production with Pichia pastoris Mut+ phenotype in continuous culture. Biotechnol Prog 31:707–714. https://doi.org/10.1002/btpr.2069
Rahimi A, Hosseini SN, Javidanbardan A, Khatami M (2019) Continuous fermentation of recombinant Pichia pastoris Mut+ producing HBsAg: Optimizing dilution rate and determining strain-specific parameters. Food Bioprod Proc 118:248–257. https://doi.org/10.1016/j.fbp.2019.09.011
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K (2017) Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14:491–502. https://doi.org/10.1038/nrgastro.2017.75
Yang Z, Zhang Z (2018) Engineering strategies for enhanced production of protein and bio-products in Pichia pastoris: a review. Biotechnol Adv 36:182–195
Che Z, Cao X, Chen G et al (2020) An effective combination of codon optimization, gene dosage, and process optimization for high-level production of fibrinolytic enzyme in Komagataella phaffii (Pichia pastoris). BMC Biotechnol 20:63. https://doi.org/10.1186/s12896-020-00654-7
Bali V, Panesar PS, Bera MB, Panesar R (2015) Fructo-oligosaccharides: production, purification and potential applications. Crit Rev Food Sci Nutr 55:1475–1490. https://doi.org/10.1080/10408398.2012.694084
Flores-Maltos DA, Mussatto SI, Contreras-Esquivel JC, Rodríguez-Herrera R, Teixeira JA, Aguilar CN (2016) Biotechnological production and application of fructooligosaccharides. Crit Rev Biotechnol 36:259–267. https://doi.org/10.3109/07388551.2014.953443
Nobre C, Alves Filho EG, Fernandes FA, Brito ES, Rodrigues S, Teixeira JA, Rodrigues LR (2018) Production of fructooligosaccharides by Aspergillus ibericus and their chemical characterization. LWT Sci Technol 89:58–64. https://doi.org/10.1016/j.lwt.2017.10.015
Peebo K, Neubauer P (2018) Application of continuous culture methods to recombinant protein production in microorganisms. Microorganisms 6:56. https://doi.org/10.3390/microorganisms6030056
Mears L, Stocks SM, Sin G, Gernaey KV (2017) A review of control strategies for manipulating the feed rate in fed-batch fermentation processes. J Biotechnol 245:34–46. https://doi.org/10.1016/j.jbiotec.2017.01.008
Gong X, Li D, Li X, Fang Q, Han X, Wu Y, Yang S, Shen BQ (2006) Fed-batch culture optimization of a growth-associated hybridoma cell line in chemically defined protein-free media. Cytotechnology 52:25–38. https://doi.org/10.1007/s10616-006-9026-3
Flikweert MT, Kuyper M, van Maris AJ, Kötter P, van Dijken JP, Pronk JT (1999) Steady-state and transient-state analysis of growth and metabolite production in a Saccharomyces cerevisiae strain with reduced pyruvate-decarboxylase activity. Biotechnol Bioeng 66(1):42–50
Herwig C, Marison IW, von Stockar U (2001) On-line stoichiometry and identification of metabolic state in dynamic process conditions. Biotechnol Bioeng 75:345–354. https://doi.org/10.1002/bit.10058
Rahimi A, Hosseini SN, Karimi A, Aghdasinia H, Mianroodi RA (2019) Enhancing the efficiency of recombinant hepatitis B surface antigen production in Pichia pastoris by employing continuous fermentation. Biochem Eng J 141:112–119. https://doi.org/10.1016/j.bej.2018.10.019
Zhang W, Liu CP, Inan M, Meagher MM (2004) Optimization of cell density and dilution rate in Pichia pastoris continuous fermentations for production of recombinant proteins. J Indust Microbiol Biotechnol 31:330–334. https://doi.org/10.1007/s10295-004-0155-4
Garrigós-Martínez J, Nieto-Taype MA, Gasset-Franch A, Montesinos-Seguí JL, Garcia-Ortega X, Valero F (2019) Specific growth rate governs AOX1 gene expression, affecting the production kinetics of Pichia pastoris (Komagataella phaffii) PAOX1-driven recombinant producer strains with different target gene dosages. Microb Cell Fact 18:187. https://doi.org/10.1186/s12934-019-1240-8
Shi L, Wang J, Wang X, Zhang Y, Song Z, Cai M, Zhou X (2019) Transcriptome and metabolome analyses reveal global behaviour of a genetically engineered methanol-independent Pichia pastoris strain. Process Biochem 76:46–54. https://doi.org/10.1016/j.procbio.2018.10.014
Niu H, Jost L, Pirlot N, Sassi H, Daukandt M, Rodriguez C, Fickers P (2013) A quantitative study of methanol/sorbitol co-feeding process of a Pichia pastoris Mut+/pAOX1-lacZ strain. Microb Cell Fact 12:33. https://doi.org/10.1186/1475-2859-12-33
Singh A, Narang A (2023) P AOX1 expression in mixed-substrate continuous cultures of Komagataella phaffii (Pichia pastoris) is completely determined by methanol consumption regardless of the secondary carbon source. Front Bioeng Biotechnol 11:1123703. https://doi.org/10.3389/fbioe.2023.1123703
Wurm DJ, Spadiut O (2019) Efficient development of a mixed feed process for Pichia pastoris. Method Mol Biol (Clifton NJ) 1923:323–333. https://doi.org/10.1007/978-1-4939-9024-5_15
Batra J, Beri D, Mishra S (2014) Response surface methodology based optimization of β-glucosidase production from Pichia pastoris. Appl Biochem Biotechnol 172:380–393. https://doi.org/10.1007/s12010-013-0519-1
Tomàs-Gamisans M, Andrade CCP, Maresca F, Monforte S, Ferrer P, Albiol J (2020) Redox engineering by ectopic overexpression of NADH kinase in recombinant Pichia pastoris (Komagataella phaffii): impact on cell physiology and recombinant production of secreted proteins. Appl Environ Microbiol 86:e02038-e2119. https://doi.org/10.1128/AEM.02038-19
Velastegui E, Quezada J, Guerrero K, Altamirano C, Martinez JA, Berrios J, Fickers P (2023) Is heterogeneity in large-scale bioreactors a real problem in recombinant protein synthesis by Pichia pastoris? Appl Microbiol Biotechnol 107:2223–2233. https://doi.org/10.1007/s00253-023-12434-2
Acknowledgements
The authors would like to acknowledge the help received from the staff members in the Bioprocess Laboratory (Department of Biochemical Engineering and Biotechnology) for reactor operations.
Funding
This work was funded through a research grant received from the Department of Biotechnology (Ministry of Science and Technology, Govt. of India), Grant number: BT/PR13813/BBE/117/59/2015). ASJ acknowledges the JRF fellowship received from the Department of Biotechnology.
Author information
Authors and Affiliations
Contributions
Avijeet S. Jaswal: conceptualization, designing, methodology, conducting experiments, data analyses, writing—first draft, final draft. Ravikrishnan Elangovan: conceptualization, bioinformatics, data analyses, final draft preparation. Saroj Mishra: designing, data analyses, project fund acquirement and management, final draft preparation. All the authors read the final version and have approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
It is declared that the article does not contain any studies conducted with human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jaswal, A.S., Elangovan, R. & Mishra, S. Optimization of dilution rate and mixed carbon feed for continuous production of recombinant plant sucrose:sucrose 1-fructosyltransferase in Komagataella phaffii. Bioprocess Biosyst Eng (2024). https://doi.org/10.1007/s00449-024-03045-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00449-024-03045-w