Abstract
Purpose
Immunotherapy with programmed cell death 1/ligand 1 (PD-1/PD-L1) checkpoint inhibitors has revolutionized the systemic treatment of solid tumors, including bladder cancer. Previous studies have shown that enhanced glycolysis, tumor-associated macrophage (TAM) infiltration, and TGF-β secretion in the tumor microenvironment (TME) are closely related to PD-1/PD-L1 inhibitor immunotherapy resistance. However, the potential mechanism of their interaction in bladder cancer has not been fully uncovered.
Methods
By coculturing bladder cancer cells and TAMs, we studied the relationship and interaction mechanism between tumor cell glycolysis, TAM functional remodeling, TGF-β positive feedback secretion, and PD-L1 mRNA m6A methylation in the bladder cancer microenvironment.
Results
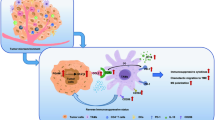
Bioinformatics analysis and IHC staining found a close correlation between tumor glycolysis, M2 TAM infiltration, and the prognosis of bladder cancer patients. In Vitro experiments demonstrated that bladder cancer cells could re-educate M2 TAMs through lactate and promote TGF-β secretion via the HIF-1α signaling pathway. Reciprocally, in vitro, and in vivo experiments validated that M2 TAMs could promote glycolysis in bladder cancer cells by TGF-β via the Smad2/3 signaling pathways. Furthermore, M2 TAMs could also promote CSCs and EMT of bladder cancer cells. More importantly, we found M2 TAMs enhance PD-L1 mRNA m6A methylation by promoting METLL3 expression in bladder cancer via the TGF-β/Smad2/3 pathway in the TME.
Conclusions
Our study highlights a feed-forward loop based on aerobic glycolysis and TGF-β between M2 TAMs and bladder cancer cells, which may be a potential mechanism of malignant progression and immunotherapy resistance in bladder cancer.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Bertero A, Brown S, Madrigal P, Osnato A, Ortmann D, Yiangou L, Kadiwala J, Hubner NC, de Los Mozos IR, Sadée C, Lenaerts AS, Nakanoh S, Grandy R, Farnell E, Ule J, Stunnenberg HG, Mendjan S, Vallier L (2018) The SMAD2/3 interactome reveals that TGFβ controls m6A mRNA methylation in pluripotency. Nature 555(7695):256–259. https://doi.org/10.1038/nature25784
Cassetta L, Pollard JW (2018) Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov 17(12):887–904. https://doi.org/10.1038/nrd.2018.169
Chanput W, Mes JJ, Wichers HJ (2014) THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol 23(1):37–45. https://doi.org/10.1016/j.intimp.2014.08.002
Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y, Tu Q, Yin D, Lin D, Wong PP, Huang D, **ng Y, Zhao J, Li M, Liu Q, Su F, Su S, Song E (2019) Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol 21(4):498–510. https://doi.org/10.1038/s41556-019-0299-0
Chen Y, Zhang J, Zhang M, Song Y, Zhang Y, Fan S, Ren S, Fu L, Zhang N, Hui H, Shen X (2021) Baicalein resensitizes tamoxifen-resistant breast cancer cells by reducing aerobic glycolysis and reversing mitochondrial dysfunction via inhibition of hypoxia-inducible factor-1α. Clin Transl Med. 11(11):e577. https://doi.org/10.1002/ctm2.577
Choo YW, Kang M, Kim HY, Han J, Kang S, Lee JR, Jeong GJ, Kwon SP, Song SY, Go S, Jung M, Hong J, Kim BS (2018) M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano 12(9):8977–8993. https://doi.org/10.1021/acsnano.8b02446
Comito G, Iscaro A, Bacci M, Morandi A, Ippolito L, Parri M, Montagnani I, Raspollini MR, Serni S, Simeoni L, Giannoni E, Chiarugi P (2019) Lactate modulates CD4+ T-cell polarization and induces an immunosuppressive environment, which sustains prostate carcinoma progression via TLR8/miR21 axis. Oncogene 38(19):3681–3695. https://doi.org/10.1038/s41388-019-0688-7
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–7. https://doi.org/10.1038/nature01322
Ding J, Yang C, Zhang Y, Wang J, Zhang S, Guo D, Yin T, Yang J (2021) M2 macrophage-derived G-CSF promotes trophoblasts EMT, invasion and migration via activating PI3K/Akt/Erk1/2 pathway to mediate normal pregnancy. J Cell Mol Med 25(4):2136–2147. https://doi.org/10.1111/jcmm.16191
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, Ring AM, Connolly AJ, Weissman IL (2017) PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545(7655):495–499. https://doi.org/10.1038/nature22396
Ippolito L, Morandi A, Giannoni E, Chiarugi P (2019) Lactate: a metabolic driver in the tumour landscape. Trends Biochem Sci 44(2):153–166. https://doi.org/10.1016/j.tibs.2018.10.011
Jiang Z, Liu Z, Li M, Chen C, Wang X (2019) Increased glycolysis correlates with elevated immune activity in tumor immune microenvironment. EBioMedicine 42:431–442. https://doi.org/10.1016/j.ebiom.2019.03.068
Kobierzycki C, Pula B, Werynska B, Piotrowska A, Muszczynska-Bernhard B, Dziegiel P, Rakus D (2014) The lack of evidence for correlation of pyruvate kinase M2 expression with tumor grade in non-small cell lung cancer. Anticancer Res 34(7):3811–3817
Lewis CE, Pollard JW (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res 66(2):605–612. https://doi.org/10.1158/0008-5472.CAN-05-4005
Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Şenbabaoğlu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Höglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T (2018) TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554(7693):544–548. https://doi.org/10.1038/nature25501
Ngwa VM, Edwards DN, Philip M, Chen J (2019) Microenvironmental metabolism regulates antitumor immunity. Cancer Res 79(16):4003–4008. https://doi.org/10.1158/0008-5472.CAN-19-0617
Ni Z, Sun P, Zheng J, Wu M, Yang C, Cheng M, Yin M, Cui C, Wang G, Yuan L, Gao Q, Li Y (2022) JNK signaling promotes bladder cancer immune escape by regulating METTL3-mediated m6A modification of PD-L1 mRNA. Cancer Res 82(9):1789–1802. https://doi.org/10.1158/0008-5472.CAN-21-1323
Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guérin M, Biton J, Ouakrim H, Régnier F, Lupo A, Alifano M, Damotte D, Donnadieu E (2018) Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc Natl Acad Sci USA 115(17):E4041–E4050. https://doi.org/10.1073/pnas.1720948115
Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141(1):39–51. https://doi.org/10.1016/j.cell.2010.03.014
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, Byrom D, Matarin JA, Calon A, Rivas EI, Nebreda AR, Riera A, Attolini CS, Batlle E (2018) TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554(7693):538–543. https://doi.org/10.1038/nature2549
Wan W, Ao X, Chen Q, Yu Y, Ao L, **ng W, Guo W, Wu X, Pu C, Hu X, Li Z, Yao M, Luo D, Xu X (2022) METTL3/IGF2BP3 axis inhibits tumor immune surveillance by upregulating N6-methyladenosine modification of PD-L1 mRNA in breast cancer. Mol Cancer 21(1):60. https://doi.org/10.1186/s12943-021-01447-y
Wang ZH, Peng WB, Zhang P, Yang XP, Zhou Q (2021) Lactate in the tumour microenvironment: from immune modulation to therapy. EBioMedicine. 73:103627. https://doi.org/10.1016/j.ebiom.2021.103627
Yu Y, Liang Y, Li D, Wang L, Liang Z, Chen Y, Ma G, Wu H, Jiao W, Niu H (2021) Glucose metabolism involved in PD-L1-mediated immune escape in the malignant kidney tumour microenvironment. Cell Death Discov 7(1):15. https://doi.org/10.1038/s41420-021-00401-7
Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, Ding J, Czyz D, Hu R, Ye Z, He M, Zheng YG, Shuman HA, Dai L, Ren B, Roeder RG, Becker L, Zhao Y (2019) Metabolic regulation of gene expression by histone lactylation. Nature 574(7779):575–580. https://doi.org/10.1038/s41586-019-1678-1
Zhang Y, Zhang X, Meng Y, Xu X, Zuo D (2022) The role of glycolysis and lactate in the induction of tumor-associated macrophages immunosuppressive phenotype. Int Immunopharmacol. 110:108994. https://doi.org/10.1016/j.intimp.2022.108994
Zhao Y, Wang D, Xu T, Liu P, Cao Y, Wang Y, Yang X, Xu X, Wang X, Niu H (2015) Bladder cancer cells re-educate TAMs through lactate shuttling in the microfluidic cancer microenvironment. Oncotarget 6(36):39196–39210. https://doi.org/10.18632/oncotarget.5538
Zheng Y, Yang W, Aldape K, He J, Lu Z (2013) Epidermal growth factor (EGF)-enhanced vascular cell adhesion molecule-1 (VCAM-1) expression promotes macrophage and glioblastoma cell interaction and tumor cell invasion. J Biol Chem 288(44):31488–31495. https://doi.org/10.1074/jbc.M113.499020
Funding
This work was supported by the National Natural Science Foundation of China (No. 81972378, 81101932).
Author information
Authors and Affiliations
Contributions
CS wrote the main manuscript text. JiL and WJ validated data. XZ and XZ was analyzed data by software. XY and YW reviewed and edited. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Affiliated Hospital of Qingdao University.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
432_2023_5164_MOESM1_ESM.tif
Supplementary 1. (A) The TCGA and GEO bladder cancer cohorts were divided into three clusters when k = 3. (B) Consensus clustering cumulative distribution function (CDK) fork = 2 to 9. (C) Relative change in area under the CDF curve for k = 2 to 9. (D) The difference in the immune cell infiltration among three glycolysis clusters by using the CIBERSORT dataset. (E, F) The difference in the cytokine-related gene expression among three glycolysis clusters by using TCGA and GEO bladder cancer cohorts. (G) The experimental scheme along with the treatment protocol (TIF 17857 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, C., Liu, J., Jiao, W. et al. A feed-forward loop based on aerobic glycolysis and TGF-β between tumor-associated macrophages and bladder cancer cells promoted malignant progression and immune escape. J Cancer Res Clin Oncol 149, 12867–12880 (2023). https://doi.org/10.1007/s00432-023-05164-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05164-5