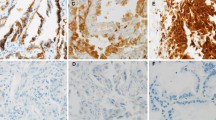
Abstract
The invasive tumor front underlies the biological aggressiveness and epithelial–mesenchymal transition (EMT) in various human malignances. However, the molecular and biological characteristics of invasive tumor front in NPC have rarely been described. Additionally, the features of cancer stem cells (CSCs) in the invasive front of tumors and its correlation with EMT also remain elusive. Our study was to investigate the expression of CSCs marker aldehyde dehydrogenase 1 (ALDH1) in the invasive front of nasopharyngeal carcinoma (NPC) and its clinical significance. Immunohistochemistry was mainly used to detect ALDH1 expression in the invasive front of NPC. The relationship between ALDH1 expression and EMT-associated markers was also examined. ALDH1 expression in the invasive front correlated strongly with lymphatic invasion (p < 0.001), T classification (p = 0.001), M classification (p < 0.001), clinical stage (p < 0.001), and local recurrence (p = 0.008). ALDH1 overexpression in the invasive front contributed to worse survival of NPC, particularly in patients with early stage (T1-T2 or N0-N1) (p < 0.001 and p = 0.002, respectively), though it was not an independent prognostic factor (p = 0.196). Furthermore, in the invasive front of NPC, ALDH1 expression correlated significantly with EMT-related biomarkers E-cadherin (p = 0.026), Vimentin (p < 0.001), Periostin (p < 0.001), and Snail (p < 0.001), but not with β-catenin (p = 0.143). Our findings demonstrate first that ALDH1 expression in the invasive front links closely with EMT characteristics and tumor aggressiveness, which might provide a useful prognostic marker for NPC patients.



Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Zhang L, Zhu YX, Wang Y, Huang CP, Wu Y, Ji QH (2011) Salvage surgery for neck residue or recurrence of nasopharyngeal carcinoma: a 10-year experience. Ann Surg Oncol 18:233–238
Teo PM, Kwan WH, Lee WY, Leung SF, Johnson PJ (1996) Prognosticators determining survival subsequent to distant metastasis from nasopharyngeal carcinoma. Cancer 77:2423–2431
Bànkfalvi A, Piffkò J (2000) Prognostic and predictive factors in oral cancer: the role of the invasive tumour front. J Oral Pathol Med 29:291–298
Rowe RG, Weiss SJ (2009) Navigating ECM barriers at the invasive front: the cancer cell–stroma interface. Annu Rev Cell Dev Biol 25:567–595
Zlobec I, Lugli A (2010) Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget 1:651–661
Piffkò J, Bànkfalvi A, Ofner D, Bryne M, Rasch D, Joos U, Böcker W, Schmid KW (1997) Prognostic value of histobiological factors (malignancy grading and AgNOR content) assessed at the invasive tumour front of oral squamous cell carcinomas. Br J Cancer 75:1543–1546
Impola U, Uitto VJ, Hietanen J, Hakkinen L, Zhang L, Larjava H, Isaka K, Saarialho-Kere U (2004) Differential expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. J Pathol 202:14–22
Fonseca I, Pereira T, Rosa-Santos J, Soares J (2001) Expression of CD44 isoforms in squamous cell carcinoma of the border of the tongue: a correlation with histological grade, pattern of stromal invasion, and cell differentiation. J Surg Oncol 76:115–120
Alpízar-Alpízar W, Christensen IJ, Santoni-Rugiu E et al (2012) Urokinase plasminogen activator receptor on invasive cancer cells: a prognostic factor in distal gastric adenocarcinoma. Int J Cancer 131:E329–E336
Wang L, Cheng H, Liu Y, Wang L, Yu W, Zhang G, Chen B, Yu Z, Hu S (2011) Prognostic value of nuclear β-catenin overexpression at invasive front in colorectal cancer for synchronous liver metastasis. Ann Surg Oncol 18:1553–1559
Kurahara H, Takao S, Kuwahata T, Nagai T, Ding Q, Maeda K, Shinchi H, Mataki Y, Maemura K, Matsuyama T, Natsugoe S (2012) Clinical significance of folate receptor β-expressing tumor-associated macrophages in pancreatic cancer. Ann Surg Oncol 19:2264–2271
Ljuslinder I, Melin B, Henriksson ML, Öberg Å, Palmqvist R (2011) Increased epidermal growth factor receptor expression at the invasive margin is a negative prognostic factor in colorectal cancer. Int J Cancer 128:2031–2037
van Kempen LC, van den Hurk K, Lazar V, Michiels S, Winnepenninckx V, Stas M, Spatz A, van den Oord JJ (2012) Loss of microRNA-200a and c, and microRNA-203 expression at the invasive front of primary cutaneous melanoma is associated with increased thickness and disease progression. Virchows Arch 461:441–448
Clevers H (2011) The cancer stem cell: premises, promises and challenges. Nat Med 17:313–319
Gupta PB, Chaffer CL, Weinberg RA (2009) Cancer stem cells: mirage or reality? Nat Med 15:1010–1012
Duester G, Mic FA, Molotkov A (2003) Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem Biol Interact 143–144:201–210
Hess DA, Meyerrose TE, Wirthlin L, Craft TP, Herrbrich PE, Creer MH, Nolta JA (2004) Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood 104:1648–1655
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1:555–567
Gong C, Yao H, Liu Q, Chen J, Shi J, Su F, Song E (2010) Markers of tumor-initiating cells predict chemoresistance in breast cancer. PLoS One 5:e15630
Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, **ng L, Wang H, Liu Z, Su Y, Stass SA, Katz RL (2009) Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res 7:330–338
Su Y, Qiu Q, Zhang X, Jiang Z, Leng Q, Liu Z, Stass SA, Jiang F (2010) Aldehyde dehydrogenase 1 A1-positive cell population is enriched in tumor-initiating cells and associated with progression of bladder cancer. Cancer Epidemiol Biomarkers Prev 19:327–337
Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W, Kopetz S, McConkey DJ, Evans DB, Gallick GE (2009) ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. Mol Cancer Ther 8:310–314
Wu A, Luo W, Zhang Q, Yang Z, Zhang G, Li S, Yao K (2013) Aldehyde dehydrogenase 1, a functional marker for identifying cancer stem cells in human nasopharyngeal carcinoma. Cancer Lett 30:181–189
Luo W, Fang W, Li S, Yao K (2012) Aberrant expression of nuclear vimentin and related epithelial-mesenchymal transition markers in nasopharyngeal carcinoma. Int J Cancer 131:1863–1873
Luo WR, Li SY, Cai LM, Yao KT (2012) High expression of nuclear snail, but not cytoplasmic staining, predicts poor survival in nasopharyngeal carcinoma. Ann Surg Oncol 19:2971–2979
Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454
Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2:76–83
Christofori G (2006) New signals from the invasive front. Nature 441:444–450
Stewart CJ, Crook ML, Little L, Louwen K (2011) Correlation between invasive pattern and immunophenotypic alterations in endocervical adenocarcinoma. Histopathology 58:720–728
Usami Y, Satake S, Nakayama F, Matsumoto M, Ohnuma K, Komori T, Semba S, Ito A, Yokozaki H (2008) Snail-associated epithelial-mesenchymal transition promotes oesophageal squamous cell carcinoma motility and progression. J Pathol 215:330–339
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715
Chen C, Wei Y, Hummel M, Hoffmann TK, Gross M, Kaufmann AM, Albers AE (2011) Evidence for epithelial–mesenchymal transition in cancer stem cells of head and neck squamous cell carcinoma. PLoS One 6:e16466
Kong D, Banerjee S, Ahmad A, Li Y, Wang Z, Sethi S, Sarkar FH (2010) Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One 5:e12445
Chang CJ, Chao CH, **a W, Yang JY, **ong Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH, Liu M, Chen CT, Yu D, Hung MC (2011) p53 regulates epithelial–mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol 13:317–323
Luo WR, Chen XY, Li SY, Wu AB, Yao KT (2012) Neoplastic spindle cells in nasopharyngeal carcinoma show features of epithelial–mesenchymal transition. Histopathology 61:113–122
Kitase Y, Yamashiro K, Fu K, Richman JM, Shuler CF (2011) Spatiotemporal localization of periostin and its potential role in epithelial–mesenchymal transition during palatal fusion. Cells Tissues Organs 193:53–63
Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J (2011) Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481:85–89
Acknowledgments
This work was supported by funding from National Natural Science Foundation of China (NSFC)-Guangdong Joint Fund [u0732006] and National Natural Science Foundation of China [81202125].
Conflict of interest statement
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Luo, Wr., Yao, Kt. Cancer stem cell characteristics, ALDH1 expression in the invasive front of nasopharyngeal carcinoma. Virchows Arch 464, 35–43 (2014). https://doi.org/10.1007/s00428-013-1508-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-013-1508-z