Abstract
Parathyroid hormone-related protein (PTHrP) released from detrusor smooth muscle (DSM) as the bladder fills acts as an endogenous DSM relaxant to facilitate bladder storage function. Here, the effects of exogenous PTHrP on transient pressure rises (TPRs) in the bladder and associated afferent nerve activity during bladder filling were investigated. In anaesthetized rats, changes in the intravesical pressure were measured while the bladder was gradually filled with saline. Afferent nerve activity was simultaneously recorded from their centrally disconnected left pelvic nerves. In DSM strips, spontaneous and nerve-evoked contractions were isometrically recorded. The distribution of PTHrP receptors (PTHrPRs) in the bladder wall was also examined by fluorescence immunostaining. The bladders in which the contralateral pelvic nerve was also centrally disconnected developed nifedipine, an L-type voltage-dependent Ca2+ channel blocker-sensitive TPRs (< 3 mmHg). Intravenous administration of PTHrP suppressed these TPRs and associated bursts of afferent nerve activity. In the bladders with centrally connected contralateral pelvic nerves, atropine, a muscarinic receptor antagonist-sensitive large TPRs (> 3 mmHg) developed in the late filling phase. PTHrP diminished the large TPRs and corresponding surges of afferent nerve activity. In DSM strips, bath-applied PTHrP (10 nM) suppressed spontaneous phasic contractions, while less affecting nerve-evoked contractions. PTHrPRs were expressed in DSM cells but not in intramural nerve fibers. Thus, PTHrP appears to suppress bladder TPRs and associated afferent nerve activity even under the influence of low degree of parasympathetic neural input during storage phases. Endogenous PTHrP may indirectly attenuate afferent nerve activity by suppressing TPRs to facilitate urinary accommodation.







Similar content being viewed by others
Data availability
Data is available on demand from Professor Hikaru Hashitani in whose laboratory this research was undertaken.
References
Moseley JM, Gillespie MT (1995) Parathyroid hormone-related protein. Crit Rev Clin Lab Sci 32:299–343. https://doi.org/10.3109/10408369509084687
Clemens TL, Cormier S, Eichinger A, Endlich K, Fiaschi-Taesch N, Fischer E, Friedman PA, Karaplis AC, Massfelder T, Rossert J, Schlüter KD, Silve C, Stewart AF, Takane K, Helwig JJ (2001) Parathyroid hormone-related protein and its receptors: nuclear functions and roles in the renal and cardiovascular systems, the placental trophoblasts and the pancreatic islets. Br J Pharmacol 134:1113–1136. https://doi.org/10.1038/sj.bjp.0704378
Jans DA, Thomas RJ, Gillespie MT (2003) Parathyroid hormone-related protein (PTHrP): a nucleocytoplasmic shuttling protein with distinct paracrine and intracrine roles. Vitam Horm 66:345–384. https://doi.org/10.1016/S0083-6729(03)01010-0
Philbrick WM, Wysolmerski JJ, Galbraith S, Holt E, Orloff JJ, Yang KH, Vasavada RC, Weir EC, Broadus AE, Stewart AF (1996) Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev 76:127–173. https://doi.org/10.1152/physrev.1996.76.1.127
Nishikawa N, Kanematsu A, Negoro H, Imamura M, Sugino Y, Okinami T, Yoshimura K, Hashitani H, Ogawa O (2013) PTHrP is endogenous relaxant for spontaneous smooth muscle contraction in urinary bladder of female rat. Endocrinology 154:2058. https://doi.org/10.1210/en.2012-2142
Yamamoto M, Harm SC, Grasser WA, Thiede MA (1992) Parathyroid hormone-related protein in the rat urinary bladder: a smooth muscle relaxant produced locally in response to mechanical stretch. Proc Natl Acad Sci U S A 89:5326. https://doi.org/10.1073/pnas.89.12.5326
Lee K, Mitsui R, Kajioka S, Naito S, Hashitani H (2016) Role of PTHrP and sensory nerve peptides in regulating contractility of muscularis mucosae and detrusor smooth muscle in the guinea pig bladder. J Urol 196:1287–1294. https://doi.org/10.1016/j.juro.2016.04.082
Biallosterski BT, van Koeveringe GA, van Kerrebroeck PE, Gillespie JI, de Wachter SG (2011) Nonvoiding activity of the guinea pig bladder. J Urol 186:721–727. https://doi.org/10.1016/j.juro.2011.03.123
Streng T, Hedlund P, Talo A, Andersson KE, Gillespie JI (2006) Phasic non-micturition contractions in the bladder of the anaesthetized and awake rat. BJU Int 97:1094–1101. https://doi.org/10.1111/j.1464-410X.2006.06137.x
Zvara P, Wright AJ, Roach K, Ursiny M, Shapiro B, Dagrosa LM, Nelson MT, Heppner TJ (2001) A non-anesthetized mouse model for recording sensory urinary bladder activity. Front Neurol 1:127. https://doi.org/10.3389/fneur.2010.00127
Gillespie JI (2004) Phosphodiesterase-linked inhibition of nonmicturition activity in the isolated bladder. BJU Int 93:1325–1332. https://doi.org/10.1111/j.1464-410X.2004.04840.x
Parsons BA, Drake MJ, Gammie A, Fry CH, Vahabi B (2012) The validation of a functional, isolated pig bladder model for physiological experimentation. Front Pharmacol 30(3):52. https://doi.org/10.3389/fphar.2012.00052
Vahabi B, Drake MJ (2015) Physiological and pathophysiological implications of micromotion activity in urinary bladder function. Acta Physiol (Oxf) 213:360–370. https://doi.org/10.1111/apha.12373
de Groat WC, Yoshimura N (2009) Afferent nerve regulation of bladder function in health and disease. Handbook Exp Pharmacol 194:91–138. https://doi.org/10.1007/978-3-540-79090-7_4
Heppner TJ, Tykocki NR, Hill-Eubanks D, Nelson MT (2016) Transient contractions of urinary bladder smooth muscle are drivers of afferent nerve activity during filling. J Gen Physiol 147:323–335. https://doi.org/10.1085/jgp.201511550
Drake MJ, Hedlund P, Harvey IJ, Pandita RK, Andersson KE, Gillespie JI (2003) Partial outlet obstruction enhances modular autonomous activity in the isolated rat bladder. J Urol 170:276–279. https://doi.org/10.1097/01.ju.0000069722.35137.e0
Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, Kanai A (2007) Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol 293(4):F1018-1025. https://doi.org/10.1152/ajprenal.00183.2007
Aizawa N, Homma Y, Igawa Y (2012) Effects of mirabegron, a novel β3-adrenoceptor agonist, on primary bladder afferent activity and bladder microcontractions in rats compared with the effects of oxybutynin. Eur Urol 62:1165–1173. https://doi.org/10.1016/j.eururo.2012.08.056
Buckner SA, Milicic I, Daza AV, Coghlan MJ, Gopalakrishnan M (2002) Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br J Pharmacol 135:639–648. https://doi.org/10.1038/sj.bjp.0704499
Guarneri L, Ibba M, Angelico P, Colombo D, Fredella B, Testa R (1993) Effects of drugs used in the therapy of detrusor hyperactivity on the volume-induced contractions of the rat urinary bladder. Pharmacol Res 27(2):173–187. https://doi.org/10.1006/phrs.1993.1017
Lluel P, Barras M, Palea S (2002) Cholinergic and purinergic contribution to the micturition reflex in conscious rats with long-term bladder outlet obstruction. Neurourol Urodyn 21(2):142–153. https://doi.org/10.1002/nau.10007
Hashitani H, Brading AF (2003) Ionic basis for the regulation of guinea-pig urinary bladder. Br J Pharmacol 141:183–193. https://doi.org/10.1038/sj.bjp.0705320
Yoshimura N, Seki S, de Groat WC (2001) Nitric oxide modulates Ca2+ channels in dorsal root ganglion neurons innervating rat urinary bladder. J Neurophysiol 86:304–311. https://doi.org/10.1152/jn.2001.86.1.304
van Asselt E, le Feber J, van Mastrigt R (1999) Threshold for efferent bladder nerve firing in the rat. Am J Physiol 276:R1819-1824. https://doi.org/10.1152/ajpregu.1999.276.6.R1819
Zagorodnyuk VP, Gregory S, Costa M, Brookes SJ, Tramontana M, Giuliani S, Maggi CA (2009) Spontaneous release of acetylcholine from autonomic nerves in the bladder. Br J Pharmacol 157:607–619. https://doi.org/10.1111/j.1476-5381.2009.00166.x
Aizawa N, Ichihara K, Fukuhara H, Fujimura T, Andersson KE, Homma Y, Igawa Y (2017) Characteristics of the mechanosensitive bladder afferent activities in relation with microcontractions in male rats with bladder outlet obstruction. Sci Rep 7:7646. https://doi.org/10.1038/s41598-017-07898-y
Aronsson P, Carlsson T, Winder M, Tobin G (2015) Cyclophosphamide-induced alterations of the micturition reflex in a novel in situ urinary bladder model in the anesthetized rat. Neurourol Urodyn 34:375–380. https://doi.org/10.1002/nau.22562
Steers WD, Broder SR, Persson K et al (1998) Mechanical stretch increases secretion of parathyroid hormone-related protein by cultured bladder smooth muscle cells. J Urol 160:908–912. https://doi.org/10.1097/00005392-199809010-00087
Bramich NJ, Brading AF (1996) Electrical properties of smooth muscle in the guinea-pig urinary bladder. J Physiol 492:185–198. https://doi.org/10.1113/jphysiol.1996.sp021300
Hayase M, Hashitani H, Kohri K, Suzuki H (2009) Role of K+ channels in regulating spontaneous activity in detrusor smooth muscle in situ in the mouse bladder. J Urol 181:2355–2365. https://doi.org/10.1016/j.juro.2009.01.013
Stenqvist J, Winder M, Carlsson T, Aronsson P, Tobin G (2017) Urothelial acetylcholine involvement in ATP-induced contractile responses of the rat urinary bladder. Eur J Pharmacol 809:253–260. https://doi.org/10.1016/j.ejphar.2017.05.023
McCarthy CJ, Ikeda Y, Skennerton D, Chakrabarty B, Kanai AJ, Jabr RI, Fry CH (2019) Characterisation of nerve-mediated ATP release from bladder detrusor muscle and its pathological implications. Br J Pharmacol 176:4720–4730. https://doi.org/10.1111/bph.14840
Hashitani H, Brading AF, Suzuki H (2004) Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141:183–193. https://doi.org/10.1038/sj.bjp.0705602
Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A (2004) Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology 63:17–23. https://doi.org/10.1016/j.urology.2003.11.003
Perez-Martinez FC, Juan YS, Lin WY, Guven A, Mannikarottu A, Levin RM (2009) Expression of parathyroid hormone-related protein in the partially obstructed and reversed rabbit bladder. Int Urol Nephrol 41:505–511. https://doi.org/10.1007/s11255-008-9485-x
Vaidyanathan S, McDicken IW, Mansour P, Singh G, Soni BM, McCreavy DT, Wlodarski B, Carron JA, Fraser WD, Sett P, Gallagher JA (2000) Parathyroid hormone-related protein (1–34) and urothelial redifferentiation in the neuropathic urinary bladder. Spinal Cord 38:546–551. https://doi.org/10.1038/sj.sc.3101044
Nishikawa N, Yago R, Yamazaki Y, Negoro H, Suzuki M, Imamura M, Toda Y, Tanabe K, Ogawa O, Kanematsu A (2015) Expression of parathyroid hormone/parathyroid hormone-related peptide receptor 1 in normal and diseased bladder detrusor muscles: a clinico-pathological study. BMC Urol 15:2. https://doi.org/10.1186/1471-2490-15-2
Shepherd AJ, Mickle AD, Kadunganattil S, Hu H, Mohapatra DP (2018) Parathyroid hormone-related peptide elicits peripheral TRPV1-dependent mechanical hypersensitivity. Front Cell Neurosci 12:38. https://doi.org/10.3389/fncel.2018.00038
Ohguchi H, Mitsui R, Imaeda K, Joh T, Hashitani H (2017) Mechanisms of PTHrP-induced inhibition of smooth muscle contractility in the guinea pig gastric antrum. Neurogastroenterol Motil. 29:(12):e13142. https://doi.org/10.1111/nmo.13142
Horiuchi N, Hongo T, Clemens TL (1991) A 7–34 analog of the parathyroid hormone-related protein has potent antagonist and partial agonist activity in vivo. Bone Miner 12:181–188. https://doi.org/10.1016/0169-6009(91)90031-t
Acknowledgements
The authors are grateful to Dr Naoki Aizawa (Dokkyo Medical University) for his dedicated support in afferent nerve recordings, and also thank Dr Richard Lang (Monash University) for his critically reading of the manuscript.
Funding
This research was funded by Grants-in-Aid for Scientific Research < KAKENHI > (C), Grant/Award Number (20K09564) to H.H.
Author information
Authors and Affiliations
Contributions
Conceptualisation: Hikaru Hashitani; methodology: Ayu Sugiura, Retsu Mitsui, and Hikaru Hashitani; formal analysis and investigation: Ayu Sugiura, Retsu Mitsui, and Hikaru Hashitani; writing — review and editing: Ayu Sugiura, Retsu Mitsui, and Hikaru Hashitani.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The procedures described have been approved by the animal experimentation ethics committee at the Nagoya City University Medical School. Consent to participate is “Not applicable” as human study is not included in this work.
Human and animal ethics
Experiments were carried out in accordance with the Care and Use of Animals in the Field of Physiological Sciences set out by the Physiological Society of Japan (2015). Human Ethics is “Not applicable” as human study is not included in this work.
Consent for publication
The authors hereby consent to publication of the Work in Pflügers Archiv European Journal of Physiology.
Competing interests
The authors declare no competing interests.
Authors’ information
Hikaru Hashitani, M.D., Ph.D., Professor, Department of Cell Physiology, Nagoya City University Graduate School of Medical Sciences, Nagoya 467–8601, Japan, Phone: + 81–52-8538131, Fax: + 81–52-8421538, Email address: hasitani@med.nagoya-cu.ac.jp.
Ayu Sugiura, M.D., Ph.D. student, Department of Cell Physiology, Nagoya City University Graduate School of Medical Sciences, Nagoya 467–8601, Japan, Phone: + 81–52-8538131, Fax: + 81–52-8421538, Email address: o1.12rosie34.ayu@gmail.com.
Retsu Mitsui, Ph.D., lecturer, Department of Cell Physiology, Nagoya City University Graduate School of Medical Sciences, Nagoya 467–8601, Japan, Phone: + 81–52-8538131, Fax: + 81–52-8421538, Email address: mitsui@med.nagoya-cu.ac.jp.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
424_2022_2736_MOESM1_ESM.jpg
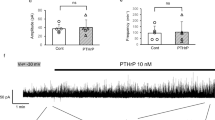
Supplementary Fig 1. The ineffectiveness of PTHrP antagonist on TPRs or afferent firing during bladder filling In centrally-disconnected bladders (N = 3), the bolus administration of PTHrP (7-34) (500 µg/kg body weight) did not affect the amplitude (a), rate of rise (b) or frequency (c) of TPRs or afferent nerve firing during bladder filling (d). Mean ± SD. n.s., PTHrP (7-34) vs. control (JPG 220 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sugiura, A., Mitsui, R. & Hashitani, H. Role of PTHrP in attenuating transient pressure rises and associated afferent nerve activity of the rat bladder. Pflugers Arch - Eur J Physiol 474, 1077–1090 (2022). https://doi.org/10.1007/s00424-022-02736-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-022-02736-1