Abstract
Background
To evaluate the relationship between the number of trinucleotide repeats (TNR) in late-onset Fuchs corneal endothelial dystrophy (FCED) and to compare the endothelial properties of FCED, first-degree relatives, and controls.
Methods
Blood samples were collected from FCEDs to determine TNR number. The FCED patients, first-degree relatives, and controls were examined with specular microscopy for central corneal thickness (CCT), endothelial cell density (ECD), pleomorphism and polymegatism, and with corneal topography for specific indicators such as (i) displacement of thinnest point of cornea, (ii) loss of isopachs, (iii) focal posterior surface depression towards anterior chamber.
Results
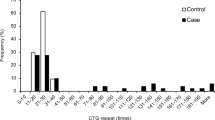
This study included 92 patients with FCED, 92 first-degree relatives, and 96 controls. CCT was thickest in FCEDs (558.0 μm) (p < 0.05) while there was no difference between relatives (533.0 μm) and controls (530.4 μm) (p = 0.845). ECD was decreased in both FCED (2069.2 mm2) and relatives (2171.4 mm2) than controls (2822.9 mm2) (p < 0.05 in both). The presence of pleomorphism and polymegatism was significant in patients with FCED (93.4% and 93.4%, respectively), relatives (86.9% and 86.04%, respectively), and controls (8.33% and 1.04%, respectively) (p < 0.05). Specific topographic indicators differed among the groups (p < 0.05). The mean repeat number of the FCED patients was 17.48 ± 4.54 (12–25) times. The TNR number of FCED cases correlated with the relative CCT (p < 0.05, R = 0.615) and cell density (p = 0.009, R = -0.499).
Conclusions
A strong association between the corneal endothelium in relatives and TNR number of FCEDs was defined. Relatives tended to have fewer corneal endothelial cells, even though they did not have clinical findings.



Similar content being viewed by others
References
OMIM Entry - # 136800 - CORNEAL DYSTROPHY, FUCHS ENDOTHELIAL, 1; FECD1. https://www.omim.org/entry/136800. Accessed 2 Mar 2022
Fuchs E (1910) Dystrophia epithelialis corneae. Albr von Graefes Arch für Ophthalmol 763(76):478–508. https://doi.org/10.1007/BF01986362
Sarnicola C, Farooq AV, Colby K (2019) Fuchs endothelial corneal dystrophy: update on pathogenesis and future directions. Eye Contact Lens 45:1–10. https://doi.org/10.1097/ICL.0000000000000469
Zoega GM, Fujisawa A, Sasaki H et al (2006) Prevalence and risk factors for cornea guttata in the Reykjavik Eye Study. Ophthalmology 113:565–569. https://doi.org/10.1016/J.OPHTHA.2005.12.014
Statistical Report - Eye Bank Association of AmericaEye Bank Association of America. https://restoresight.org/what-we-do/publications/statistical-report/. Accessed 2 Mar 2022
Xu TT, Li YJ, Afshari NA et al (2021) Disease expression and familial transmission of fuchs endothelial corneal dystrophy with and without CTG18.1 expansion. Invest Ophthalmol Vis Sci 62:17–17. https://doi.org/10.1167/IOVS.62.1.17
Porter S, Sightsavers (2017) Cataract surgical rates. Community Eye Health 30:88
Mootha VV, Gong X, Ku HC, **ng C (2014) Association and familial segregation of CTG18.1 trinucleotide repeat expansion of TCF4 gene in Fuchs’ endothelial corneal dystrophy. Invest Ophthalmol Vis Sci 55:33–42. https://doi.org/10.1167/IOVS.13-12611
Baratz KH, Tosakulwong N, Ryu E et al (2010) E2-2 protein and Fuchs’s corneal dystrophy. N Engl J Med 363:1016–1024. https://doi.org/10.1056/NEJMOA1007064
Wieben ED, Aleff RA, Tosakulwong N et al (2012) A common trinucleotide repeat expansion within the transcription factor 4 (TCF4, E2-2) gene predicts Fuchs corneal dystrophy. PLoS One 7. https://doi.org/10.1371/JOURNAL.PONE.0049083
Kuot A, Hewitt AW, Snibson GR et al (2017) TGC repeat expansion in the TCF4 gene increases the risk of Fuchs’ endothelial corneal dystrophy in Australian cases. PLoS One 12. https://doi.org/10.1371/JOURNAL.PONE.0183719
Nakano M, Okumura N, Nakagawa H et al (2015) Trinucleotide repeat expansion in the TCF4 gene in Fuchs’ endothelial corneal dystrophy in Japanese. Invest Ophthalmol Vis Sci 56:4865–4869. https://doi.org/10.1167/IOVS.15-17082
Thalamuthu A, Khor CC, Venkataraman D et al (2011) Association of TCF4 gene polymorphisms with Fuchs’ corneal dystrophy in the Chinese. Invest Ophthalmol Vis Sci 52:5573–5578. https://doi.org/10.1167/IOVS.11-7568
**ng C, Gong X, Hussain I et al (2014) Transethnic replication of association of CTG18.1 repeat expansion of TCF4 gene with Fuchs’ corneal dystrophy in Chinese implies common causal variant. Invest Ophthalmol Vis Sci 55:7073–7078. https://doi.org/10.1167/IOVS.14-15390
Soliman AZ, **ng C, Radwan SH et al (2015) Correlation of severity of Fuchs endothelial corneal dystrophy with triplet repeat expansion in TCF4. JAMA Ophthalmol 133:1386–1391. https://doi.org/10.1001/JAMAOPHTHALMOL.2015.3430
Treangen TJ, Salzberg SL (2011) Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nat Rev Genet 13:36–46. https://doi.org/10.1038/NRG3117
Ardui S, Ameur A, Vermeesch JR, Hestand MS (2018) Single molecule real-time (SMRT) sequencing comes of age: applications and utilities for medical diagnostics. Nucleic Acids Res 46:2159–2168. https://doi.org/10.1093/NAR/GKY066
Gomes-Pereira M, Monckton DG (2004) Mouse tissue culture models of unstable triplet repeats. Methods Mol Biol 277:215–227. https://doi.org/10.1385/1-59259-804-8:215
Kohwi Y, McMurray CT (2013) Trinucleotide Repeat Protocols, vol 1010. https://doi.org/10.1007/978-1-62703-411-1
Gabrieli T, Sharim H, Fridman D et al (2018) Selective nanopore sequencing of human BRCA1 by Cas9-assisted targeting of chromosome segments (CATCH). Nucleic Acids Res 46:e87. https://doi.org/10.1093/NAR/GKY411
Ebbert MTW, Farrugia SL, Sens JP et al (2018) Long-read sequencing across the C9orf72 “GGGGCC” repeat expansion: implications for clinical use and genetic discovery efforts in human disease. Mol Neurodegener 13. https://doi.org/10.1186/S13024-018-0274-4
Pham TT, Yin J, Eid JS et al (2016) Single-locus enrichment without amplification for sequencing and direct detection of epigenetic modifications. Mol Gen Genomics 291:1491–1504. https://doi.org/10.1007/S00438-016-1167-2
Höijer I, Tsai YC, Clark TA et al (2018) Detailed analysis of HTT repeat elements in human blood using targeted amplification-free long-read sequencing. Hum Mutat 39:1262–1272. https://doi.org/10.1002/HUMU.23580
Tsai Y-C, Greenberg D, Powell J et al (2017) Amplification-free, CRISPR-Cas9 targeted enrichment and SMRT sequencing of repeat-expansion disease causative genomic regions. BioRxiv:203919. https://doi.org/10.1101/203919
Sun SY, Wacker K, Baratz KH, Patel SV (2019) Determining subclinical edema in Fuchs endothelial corneal dystrophy: revised classification using Scheimpflug Tomography for Preoperative Assessment. Ophthalmology 126:195–204. https://doi.org/10.1016/J.OPHTHA.2018.07.005
Li H (2018) Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34:3094–3100. https://doi.org/10.1093/BIOINFORMATICS/BTY191
Vasanth S, Eghrari AO, Gapsis BC et al (2015) Expansion of CTG18.1 trinucleotide repeat in TCF4 Is a potent driver of Fuchs’ corneal dystrophy. Invest Ophthalmol Vis Sci 56:4531–4536. https://doi.org/10.1167/IOVS.14-16122
Eghrari AO, Vasanth S, Wang J et al (2017) CTG18.1 expansion in TCF4 ıncreases likelihood of transplantation in Fuchs corneal dystrophy. Cornea 36:40–43. https://doi.org/10.1097/ICO.0000000000001049
Shneor E, Frucht-Pery J, Granit E, Gordon-Shaag A (2020) The prevalence of corneal abnormalities in first-degree relatives of patients with keratoconus: a prospective case-control study. Ophthalmic Physiol Opt 40:442–451. https://doi.org/10.1111/OPO.12706
Jiang X, Zhang H (2021) Deterioration of Avellino corneal dystrophy in a Chinese family after LASIK. Int J Ophthalmol 14:795–799. https://doi.org/10.18240/IJO.2021.06.02
Awwad ST, Yehia M, Mehanna CJ et al (2019) Tomographic and refractive characteristics of pediatric first-degree relatives of keratoconus patients. Am J Ophthalmol 207:71–76. https://doi.org/10.1016/J.AJO.2019.05.032
Hafford-Tear NJ, Tsai YC, Sadan AN et al (2019) CRISPR/Cas9-targeted enrichment and long-read sequencing of the Fuchs endothelial corneal dystrophy–associated TCF4 triplet repeat. Genet Med 21:2092–2102. https://doi.org/10.1038/s41436-019-0453-x
Wieben ED, Aleff RA, Basu S et al (2019) Amplification-free long-read sequencing of TCF4 expanded trinucleotide repeats in Fuchs endothelial corneal dystrophy. PLoS One 14:1–14. https://doi.org/10.1371/journal.pone.0219446
Breschel TS, McInnis MG, Margolis RL et al (1997) A novel, heritable, expanding CTG repeat in an intron of the SEF2-1 gene on chromosome 18q21.1. Hum Mol Genet 6:1855–1863. https://doi.org/10.1093/HMG/6.11.1855
Riazuddin SA, McGlumphy EJ, Yeo WS et al (2011) Replication of the TCF4 intronic variant in late-onset Fuchs corneal dystrophy and evidence of independence from the FCD2 locus. Invest Ophthalmol Vis Sci 52:2825–2829. https://doi.org/10.1167/IOVS.10-6497
Li YJ, Minear MA, Rimmler J et al (2011) Replication of TCF4 through association and linkage studies in late-onset Fuchs endothelial corneal dystrophy. PLoS One 6. https://doi.org/10.1371/JOURNAL.PONE.0018044
Kumar KR, Cowley MJ, Davis RL (2019) Next-generation sequencing and emerging technologieS. Semin Thromb Hemost 45:661–673. https://doi.org/10.1055/s-0039-1688446
Clarke J, Wu HC, Jayasinghe L et al (2009) Continuous base identification for single-molecule nanopore DNA sequencing. Nat Nanotechnol 4:265–270. https://doi.org/10.1038/NNANO.2009.12
Liu Q, Zhang P, Wang D et al (2017) Interrogating the “unsequenceable” genomic trinucleotide repeat disorders by long-read sequencing. Genome Med 9:1–16. https://doi.org/10.1186/S13073-017-0456-7/FIGURES/6
Sobottka Ventura AC, Wälti R, Böhnke M (2001) Corneal thickness and endothelial density before and after cataract surgery. Br J Ophthalmol 85:18–20. https://doi.org/10.1136/BJO.85.1.18
Walkow T, Anders N, Klebe S (2000) Endothelial cell loss after phacoemulsification: relation to preoperative and intraoperative parameters. J Cataract Refract Surg 26:727–732. https://doi.org/10.1016/S0886-3350(99)00462-9
Traish AS, Colby KA (2010) Approaching cataract surgery in patients with fuchs’ endothelial dystrophy. Int Ophthalmol Clin 50:1–11. https://doi.org/10.1097/IIO.0B013E3181C5728F
Patel SV (2021) Imaging Fuchs endothelial corneal dystrophy in clinical practice and clinical trials. Cornea 40:1505–1511. https://doi.org/10.1097/ICO.0000000000002738
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Gozde SAHIN VURAL and Hilmi BOLAT. The first draft of the manuscript was written by Gozde SAHIN VURAL, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Balıkesir University Ethical Committee, Approval reference number:2021/135 and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of ınterest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study has not been presented at a conference or meeting.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahın Vural, G., Bolat, H. Nanopore sequencing method for CTG18.1 expansion in TCF4 in late-onset Fuchs endothelial corneal dystrophy and a comparison of the structural features of cornea with first-degree relatives. Graefes Arch Clin Exp Ophthalmol 262, 903–911 (2024). https://doi.org/10.1007/s00417-023-06243-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06243-6