Abstract
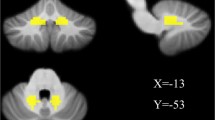
Parkinson’s disease (PD) is a progressive neurological degenerative disorder characterized by impaired motor function and non-motor dysfunctions. While recent studies have highlighted the role of the cerebellum in PD, our understanding of its role in PD remains limited. In the present study, we used resting-state fMRI to evaluate dysfunctions within the cerebellum in PD patients treated with medication and drug-naïve PD patients. We applied amplitude of low-frequency fluctuation (ALFF) and degree centrality (DC) analysis methods. Thirty-one patients with early stage PD (22 drug-naïve and 9 medicated patients) and 31 gender- and age-matched healthy controls were recruited in this study. ALFFs increased in the left cerebellar areas (lobules VI/VIIb/CruI/CruII and the dentate gyrus) and right cerebellar areas (lobules VI/VIIb/VIIIa/CruI/CruII and the dentate gyrus) of all PD patients and in the left and right cerebellar areas (lobules VI/VIIb/CruI and the dentate gyrus) of drug-naive PD patients but were not significantly changed in medicated PD patients. DC increased in the right cerebellar areas of all PD patients and medicated PD patients. All PD patients and all drug-naive PD patients showed significantly weaker functional connectivity (FC) between the left cerebellum and the left medial frontal gyrus. However, FC was significantly stronger between the right cerebellum and the left precentral and right middle occipital gyri in the medicated PD patients than in controls. Furthermore, a correlation analyses revealed that ALFF z scores in the left cerebellum (lobule VI) and right cerebellum (lobule VI/CruI and dentate gyrus) were negatively correlated with Mini-Mental State Examination (MMSE) scores in all PD patients and drug-naive patients. These results indicate that the cerebellum plays an important role in PD, mainly by exerting a compensatory effect in early stage PD. Additionally, antiparkinsonian medication would modified PD-induced changes in local neural activity and FC in PD patients. The results of this study offer novel insights into the roles of the cerebellum in early stage drug-naïve PD.


Similar content being viewed by others
References
Bedard P, Sanes JN (2009) On a basal ganglia role in learning and rehearsing visual–motor associations. Neuroimage 47:1701–1710
Benninger DH, Thees S, Kollias SS, Bassetti CL, Waldvogel D (2009) Morphological differences in Parkinson’s disease with and without rest tremor. J Neurol 256:256–263
Borghammer P, Ostergaard K, Cumming P, Gjedde A, Rodell A, Hall N, Chakravarty MM (2010) A deformation-based morphometry study of patients with early-stage Parkinson’s disease. Eur J Neurol 17:314–320
Bostan AC, Dum RP, Strick PL (2010) The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA 107:8452–8456
Bostan AC, Strick PL (2018) The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 19:338–350
Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA (2009) Cortical hubs revealed by intrinsic functional connectivity: map**, assessment of stability, and relation to Alzheimer’s disease. J Neurosci Off J Soc Neurosci 29:1860–1873
Camicioli R, Gee M, Bouchard TP, Fisher NJ, Hanstock CC, Emery DJ, Martin WR (2009) Voxel-based morphometry reveals extra-nigral atrophy patterns associated with dopamine refractory cognitive and motor impairment in Parkinsonism. Parkinsonism Relat Disord 15:187–195
Chen CH, Fremont R, Arteaga-Bracho EE, Khodakhah K (2014) Short latency cerebellar modulation of the basal ganglia. Nat Neurosci 17:1767–1775
Diedrichsen J (2006) A spatially unbiased atlas template of the human cerebellum. Neuroimage 33:127–138
Festini SB, Bernard JA, Kwak Y, Peltier S, Bohnen NI, Muller ML, Dayalu P, Seidler RD (2015) Altered cerebellar connectivity in Parkinson’s patients ON and OFF L-DOPA medication. Front Hum Neurosci 9:214
Hu X, Zhang J, Jiang X, Zhou C, Wei L, Yin X, Wu Y, Li J, Zhang Y, Wang J (2015) Decreased interhemispheric functional connectivity in subtypes of Parkinson’s disease. J Neurol 262:760–767
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79:368–376
Ji GJ, Hu P, Liu TT, Li Y, Chen X, Zhu C, Tian Y, Chen X, Wang K (2018) Functional connectivity of the corticobasal ganglia–thalamocortical network in Parkinson disease: a systematic review and meta-analysis with cross-validation. Radiology 287:973–982
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912
Lewis MM, Galley S, Johnson S, Stevenson J, Huang X, McKeown MJ (2013) The role of the cerebellum in the pathophysiology of Parkinson’s disease. Can J Neurol Sci Le journal canadien des sciences neurologiques 40:299–306
Liu H, Edmiston EK, Fan G, Xu K, Zhao B, Shang X, Wang F (2013) Altered resting-state functional connectivity of the dentate nucleus in Parkinson’s disease. Psychiatry Res 211:64–71
Middleton FA, Strick PL (2000) Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev 31:236–250
Mirdamadi JL (2016) Cerebellar role in Parkinson’s disease. J Neurophysiol 116:917–919
Nishio Y, Hirayama K, Takeda A, Hosokai Y, Ishioka T, Suzuki K, Itoyama Y, Takahashi S, Mori E (2010) Corticolimbic gray matter loss in Parkinson’s disease without dementia. Eur J Neurol 17:1090–1097
O’Callaghan C, Hornberger M, Balsters JH, Halliday GM, Lewis SJ, Shine JM (2016) Cerebellar atrophy in Parkinson’s disease and its implication for network connectivity. Brain J Neurol 139:845–855
Pan P, Zhan H, **a M, Zhang Y, Guan D, Xu Y (2017) Aberrant regional homogeneity in Parkinson’s disease: a voxel-wise meta-analysis of resting-state functional magnetic resonance imaging studies. Neurosci Biobehav Rev 72:223–231
Pelzer EA, Hintzen A, Goldau M, von Cramon DY, Timmermann L, Tittgemeyer M (2013) Cerebellar networks with basal ganglia: feasibility for tracking cerebello-pallidal and subthalamo-cerebellar projections in the human brain. Eur J Neurosci 38:3106–3114
Rascol O, Sabatini U, Fabre N, Brefel C, Loubinoux I, Celsis P, Senard JM, Montastruc JL, Chollet F (1997) The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain J Neurol 120(Pt 1):103–110
Schindlbeck KA, Eidelberg D (2018) Network imaging biomarkers: insights and clinical applications in Parkinson’s disease. Lancet Neurol 17:629–640
Simioni AC, Dagher A, Fellows LK (2016) Compensatory striatal–cerebellar connectivity in mild-moderate Parkinson’s disease. NeuroImage Clin 10:54–62
Skidmore FM, Yang M, Baxter L, von Deneen KM, Collingwood J, He G, White K, Korenkevych D, Savenkov A, Heilman KM, Gold M, Liu Y (2013) Reliability analysis of the resting state can sensitively and specifically identify the presence of Parkinson disease. Neuroimage 75:249–261
Stoodley CJ, Schmahmann JD (2010) Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46:831–844
Stoodley CJ, Schmahmann JD (2009) Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44:489–501
Sveinbjornsdottir S (2016) The clinical symptoms of Parkinson’s disease. J Neurochem 139(Suppl 1):318–324
Tahmasian M, Bettray LM, van Eimeren T, Drzezga A, Timmermann L, Eickhoff CR, Eickhoff SB, Eggers C (2015) A systematic review on the applications of resting-state fMRI in Parkinson’s disease: does dopamine replacement therapy play a role? Cortex 73:80–105
Tahmasian M, Eickhoff SB, Giehl K, Schwartz F, Herz DM, Drzezga A, van Eimeren T, Laird AR, Fox PT, Khazaie H, Zarei M, Eggers C, Eickhoff CR (2017) Resting-state functional reorganization in Parkinson’s disease: an activation likelihood estimation meta-analysis. Cortex 92:119–138
Timmann D, Drepper J, Frings M, Maschke M, Richter S, Gerwig M, Kolb FP (2010) The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex 46:845–857
Vo A, Sako W, Fujita K, Peng S, Mattis PJ, Skidmore FM, Ma Y, Ulug AM, Eidelberg D (2017) Parkinson’s disease-related network topographies characterized with resting state functional MRI. Hum Brain Mapp 38:617–630
Wu T, Hallett M (2013) The cerebellum in Parkinson’s disease. Brain J Neurol 136:696–709
Wu T, Hallett M (2005) A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain J Neurol 128:2250–2259
Wu T, Long X, Zang Y, Wang L, Hallett M, Li K, Chan P (2009) Regional homogeneity changes in patients with Parkinson’s disease. Hum Brain Mapp 30:1502–1510
Wu T, Ma Y, Zheng Z, Peng S, Wu X, Eidelberg D, Chan P (2015) Parkinson’s disease-related spatial covariance pattern identified with resting-state functional MRI. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 35:1764–1770
Wu T, Wang L, Hallett M, Li K, Chan P (2010) Neural correlates of bimanual anti-phase and in-phase movements in Parkinson’s disease. Brain J Neurol 133:2394–2409
Yan CG, Wang XD, Zuo XN, Zang YF (2016) DPABI: data processing and analysis for (resting-state) brain imaging. Neuroinformatics 14:339–351
Yu H, Sternad D, Corcos DM, Vaillancourt DE (2007) Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 35:222–233
Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF (2007) Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29:83–91
Zeighami Y, Ulla M, Iturria-Medina Y, Dadar M, Zhang Y, Larcher KM, Fonov V, Evans AC, Collins DL, Dagher A (2015) Network structure of brain atrophy in de novo Parkinson’s disease. eLife 4:e08440
Zhang K, Yu C, Zhang Y, Wu X, Zhu C, Chan P, Li K (2011) Voxel-based analysis of diffusion tensor indices in the brain in patients with Parkinson’s disease. Eur J Radiol 77:269–273
Zuo XN, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O, Milham MP (2012) Network centrality in the human functional connectome. Cereb Cortex 22:1862–1875
Acknowledgements
This research was supported by Grants from the National Natural Science Foundation of China (nos. 81571658 to X. X. Du and 81271302 to J. R. Liu), Shanghai Pujiang Program (18PJD023 to W. Chen), a research innovation project from Shanghai Municipal Science and Technology Commission (no. 14JC1404300 to J. R. Liu), the “Prevention and Control of Chronic Diseases Project” of Shanghai Hospital Development Center (no. SHDC12015310 to J. R. Liu), a project from the SHSMU-ION Research Center for Brain Disorders (no. 2015NKX006 to J. R. Liu), a project from the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (no. 20161422 to J. R. Liu), the Clinical Research Project from Shanghai Jiao Tong University School of Medicine (no. DLY201614 to J. R. Liu), and the Biomedicine Key Program from Shanghai Municipal Science and Technology Commission (no. 16411953100 to J. R. Liu). We thank the patients and their families who participated in the study for their support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
This study was carried out in accordance with the recommendations of the East China of Normal University Ethics Committee and the Independent Ethics Committee of Shanghai Ninth People’s Hospital. The study was approved by the East China of Normal University Ethics Committee (HR062-2018) and the Independent Ethics Committee of Shanghai Ninth People’s Hospital (2016-44-T1). All subjects provided signed written informed consent documents approved by the East China of Normal University Ethics Committee and the Independent Ethics Committee of Shanghai Ninth People’s Hospital.
Rights and permissions
About this article
Cite this article
Xu, S., He, XW., Zhao, R. et al. Cerebellar functional abnormalities in early stage drug-naïve and medicated Parkinson’s disease. J Neurol 266, 1578–1587 (2019). https://doi.org/10.1007/s00415-019-09294-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09294-0