Abstract
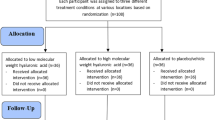
To overcome interruption of skin barrier in transdermal drug delivery, the microneedle (MN) patch penetrates the barrier by punching with its MNs. Setting a needleless patch (NL patch) as the control intervention, this study assessed the efficacy of a biodegradable hyaluronic acid MN patch (BHMN patch) for atopic dermatitis (AD) patients with dry skin. Similar two AD lesions were selected from the extremities of a participant. For one lesion, a BHMN patch was attached for 6–8 h on where an aroma cream was applied (BHMN patch group). Simultaneously, an NL patch was attached on the other lesion as in the BHMN patch group (NL patch group). For 2 weeks, the interventions were conducted 3 times a week. The local scoring AD (L-SCORAD) index, the visual analog scale for pruritus and skin dryness, skin hydration, the transepidermal water loss (TEWL), and safety were assessed. Fifteen participants finished this trial with no dropouts. Both groups improved the L-SCORAD index after 2 weeks (p < 0.05), but the score of the BHMN patch group decreased more than that of the NL patch group (p < 0.05). The other outcomes, except for the TEWL, also showed statistical significance in intragroup comparisons. Nevertheless, none of the other outcomes showed statistical significance in intergroup comparisons. The TEWL showed no statistical significance even in intragroup comparison. Recoverable minor adverse events were reported in three cases. Considering the result of L-SCORAD index, the BHMN patch may be effective for ameliorating AD. However, a large-scale confirmatory trial is necessary to reassess other outcomes.
Trial Registration: This study was registered with the Clinical Research Information Service, Republic of Korea (Submitted date: 04/01/2022, Registered date: 23/02/2022, The first participant enrollment: 01/12/2021, Registration No. KCT0007037).








Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AD:
-
Atopic dermatitis
- SC:
-
Stratum corneum
- TEWL:
-
Transepidermal water loss
- TDD:
-
Transdermal drug delivery
- MN:
-
Microneedle
- BHMN patch:
-
Biodegradable hyaluronic acid MN patch
- NL patch:
-
Needleless patch
- NDSUKH:
-
Naju Dongshin University Korean Medicine Hospital
- L-SCORAD:
-
Local Scoring Atopic Dermatitis
- VAS:
-
Visual analog scale
- SCORAD:
-
Scoring atopic dermatitis
References
Das P, Mounika P, Yellurkar ML et al (2022) Keratinocytes: an enigmatic factor in atopic dermatitis. Cells 11:1683
Guttman-Yassky E, Waldman A, Ahluwalia J et al (2017) Atopic dermatitis: pathogenesis. Semin Cutan Med Surg 36:100–103
Prausnitz MR, Elias PM, Franz TJ et al (2012) Skin barrier and transdermal drug delivery. In: Bolognia JL, Schaffer JV (eds) Dermatology, 3rd edn. Saunders Publishing, Philadelphia, pp 2065–2074
Min DL, Park EJ, Kang KH (2013) Review of clinical and experimental studies on external application treatment for atopic dermatitis in the Korean literature. The Journal of Korean Oriental Pediatrics 27:36–49
Wong ITY, Tsuyuki RT, Cresswell-Melville A et al (2017) Guidelines for the management of atopic dermatitis (eczema) for pharmacists. Can Pharm J 150:285–297
Leung DY, Guttman-Yassky E (2014) Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol 134:769–779
Tagami H, Kobayashi H, O’Goshi K, Kikuchi K (2006) Atopic xerosis: employment of noninvasive biophysical instrumentation for the functional analyses of the mildly abnormal stratum corneum and for the efficacy assessment of skin care products. J Cosmet Dermatol 5:140–149
Varothai S, Nitayavardhana S, Kulthanan K (2013) Moisturizers for patients with atopic dermatitis. Asian Pac J Allergy Immunol 31:91–98
Martanto W, Davis SP, Holiday NR et al (2004) Transdermal delivery of insulin using microneedles in vivo. Pharm Res 21:947–952
Thomas BJ, Finnin BC (2004) The transdermal revolution. Drug Discov Today 9:697–703
Prausnitz MR, Mitragotri S, Langer R (2004) Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov 3:115–124
Gerstel MS, Place VA (1976) Drug delivery device. US Patent No. 3964482
Bariya SH, Gohel MC, Mehta TA, Sharma OP (2012) Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol 64:11–29
Choi SY, Kwon HJ, Ahn GR et al (2017) Hyaluronic acid microneedle patch for the improvement of crow’s feet wrinkles. Dermatol Ther 30:e12546
Li J, Zeng M, Shan H, Tong C (2017) Microneedle patches as drug and vaccine delivery platform. Curr Med Chem 24:2413–2422
Shin JU, Kim JD, Kim HK et al (2018) The use of biodegradable microneedle patches to increase penetration of topical steroid for prurigo nodularis. Eur J Dermatol 28:71–77
Lee JH, Jung YS, Kim GM, Bae JM (2018) A hyaluronic acid-based microneedle patch to treat psoriatic plaques: a pilot open trial. Br J Dermatol 178:e24–e25
Song JH (2022) Efficacy of a biodegradable hyaluronic acid microneedle patch for dry skin in atopic dermatitis patients. Dongshin University
Julious SA (2005) Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 4:287–291
Hanifin JM, Rajka G (1980) Diagnostic features of atopic dermatitis. Acta Derm Venereol 92:44–47
Kim HW (2014) A clinical comparison study of the efficacy of moisturizer containing licochalcone A and 1% hydrocortisone in mild to moderate atopic dermatitis. The Graduate School of Hanyang University
Stettler H, Kurka P, Kandzora J et al (2017) A new topical panthenol-containing emollient for maintenance treatment of childhood atopic dermatitis: results from a multicenter prospective study. J Dermatolog Treat 28:774–779
Reich A, Heisig M, Phan NQ et al (2012) Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol 92:497–501
Lee SS, Kim SY, Im M et al (2011) The influence of physiologic lipid containing moisturizer on the normal skin barrier. Korean J Dermatolo 49:339–344
Consensus Report of the European Task Force on Atopic Dermatitis (1993) Severity scoring of atopic dermatitis: the SCORAD index. Dermatology 186:23–31
Schmitt J, Langan S, Williams HC (2007) What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol 120:1389–1398
Irvine AD, McLean WH, Leung DY (2011) Filaggrin mutations associated with skin and allergic diseases. N Engl J Med 365:1315–1327
Margolis DJ, Apter AJ, Gupta J et al (2012) The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol 130:912–917
Elias PM, Hatano Y, Williams ML (2008) Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol 121:1337–1343
Engebretsen KA, Johansen JD, Kezic S et al (2016) The effect of environmental humidity and temperature on skin barrier function and dermatitis. J Eur Acad Dermatol Venereol 30:223–249
Kim YM, Kim J, Han Y et al (2017) Short-term effects of weather and air pollution on atopic dermatitis symptoms in children: a panel study in Korea. PLoS ONE 12:e0175229
Niebuhr M, Mamerow D, Heratizadeh A et al (2011) Staphylococcal α-toxin induces a higher T cell proliferation and interleukin-31 in atopic dermatitis. Int Arch Allergy Immunol 156:412–415
Howell MD, Wollenberg A, Gallo RL et al (2006) Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol 117:836–841
Ong PY, Ohtake T, Brandt C et al (2002) Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 347:1151–1160
Kong HH, Oh J, Deming C et al (2012) Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 22:850–859
Niebuhr M, Scharonow H, Gathmann M et al (2010) Staphylococcal exotoxins are strong inducers of IL-22: A potential role in atopic dermatitis. J Allergy Clin Immunol 126:1176–83.e4
Thepen T, Langeveld-Wildschut EG, Bihari IC et al (1996) Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial TH2 response to a TH1 response in situ: an immunocytochemical study. J Allergy Clin Immunol 97:828–837
Kim JD, Jeong DH (2018) Dissolvable microneedles: applications and opportunities. ONdrugDelivery Magazine 24–29
Saeki H, Furue M, Furukawa F et al (2009) Guidelines for management of atopic dermatitis. J Dermatol 36:563–577
Miller DW, Koch SB, Yentzer BA et al (2011) An over-the-counter moisturizer is as clinically effective as, and more cost-effective than, prescription barrier creams in the treatment of children with mild-to-moderate atopic dermatitis: a randomized, controlled trial. J Drugs Dermatol 10:531–537
Giam YC, Hebert AA, Dizon MV et al (2016) A review on the role of moisturizers for atopic dermatitis. Asia Pac Allergy 6:120–128
Koh KJ, Pearce AL, Marshman G et al (2002) Tea tree oil reduces histamine-induced skin inflammation. Br J Dermatol 147:1212–1217
Seo YM, Jeong SH (2015) Effects of blending oil of lavender and thyme on oxidative stress, immunity, and skin condition in atopic dermatitis induced mice. J Korean Acad Nurs 45:367–377
Choi SM, Lee BM (2015) Safety and risk assessment of ceramide 3 in cosmetic products. Food Chem Toxicol 84:8–17
Sugarman JL, Parish LC (2009) Efficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J Drugs Dermatol 8:1106–1111
Kim SK, Karadeniz F (2012) Biological importance and applications of squalene and squalane. Adv Food Nutr Res 65:223–233
Kim DI, Kim SH, Ahn IS, Choi MS (2014) Analysis of patent information for the development of korea traditional herbal formulation for acne and that of functional cosmetics. J Korean Obstet Gynecol 27:104–115
Zink A, Traidl-Hoffmann C (2015) Green tea in dermatology—myths and facts. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 13:768–775
Seok J, Hong JY, Choi SY et al (2016) A potential relationship between skin hydration and stamp-type microneedle intradermal hyaluronic acid injection in middle-aged male face. J Cosmet Dermatol 15:578–582
Park KY, Jang WS, Lim YY et al (2013) Safety evaluation of stamp type digital microneedle devices in hairless mice. Ann Dermatol 25:46–53
Zhou CP, Liu YL, Wang HL et al (2010) Transdermal delivery of insulin using microneedle rollers in vivo. Int J Pharm 392:127–133
Oranje AP, Verbeek R, Verzaal P et al (2009) Wet-wrap treatment using dilutions of tacrolimus ointment and fluticasone propionate cream in human APOC1 (+/+) mice with atopic dermatitis. Br J Dermatol 160:54–61
Funding
This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (Grant No.: HI19C0553).
Author information
Authors and Affiliations
Contributions
Conceptualization: S-YP; methodology: S-YP; formal analysis: GL; investigation: J-HS and S-YP; resources: DHJ; writing—original draft preparation: J-HS; writing—review and editing: S-YP and J-HS; visualization: J-HS; supervision: EJA, CYS, and S-YP; project administration: S-YP and DHJ; funding acquisition: DHJ. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was conducted according to the guidelines of the Declaration of Helsinki, and it was approved by the Institutional Review Board of the Naju Dongshin University Korean Medicine Hospital (Approval No. NJ-IRB-010). Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, JH., An, E.J., Sung, C.Y. et al. A comparative study on a biodegradable hyaluronic acid microneedle patch with a needleless patch for dry skin in atopic dermatitis: a single-blinded, split-body, randomized controlled trial. Arch Dermatol Res 315, 569–581 (2023). https://doi.org/10.1007/s00403-022-02400-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-022-02400-9