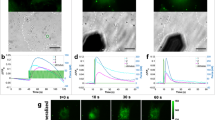
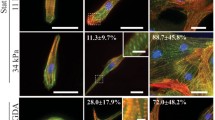
Abstract
Recognizing that cells “feel” and respond to their mechanical environment, recent studies demonstrate that many cells exhibit a phenomenon of “mechanical memory” in which features induced by prior mechanical cues persist after the mechanical stimulus has ceased. While there is a general recognition that different cell types exhibit different responses to changes in extracellular matrix stiffening, the phenomenon of mechanical memory within myocardial cell types has received little attention to date. To probe the dynamics of mechanical memory in cardiac fibroblasts (CFs) and cardiomyocytes derived from human induced pluripotent stem cells (iPSC-CMs), we employed a magnetorheological elastomer (MRE) cell culture substrate with tunable and reversible stiffness spanning the range from normal to diseased myocardium. In CFs, using increased cell area and increases in α-smooth muscle actin as markers of cellular responses to matrix stiffening, we found that induction of mechanical memory required seven days of stiff priming. Both induction and maintenance of persistent CF activation were blocked with the F-actin inhibitor cytochalasin D, while inhibitors of microtubule detyrosination had no impact on CFs. In iPSC-CMs, mechanical memory was invoked after only 24 h of stiff priming. Moreover, mechanical memory induction and maintenance were microtubule-dependent in CMs with no dependence on F-actin. Overall, these results identify the distinct temporal dynamics of mechanical memory in CFs and iPSC-CMs with different cytoskeletal mediators responsible for inducing and maintaining the stiffness-activated phenotype. Due to its flexibility, this model is broadly applicable to future studies interrogating mechanotransduction and mechanical memory in the heart and might inform strategies for attenuating the impact of load-induced pathology and excess myocardial stiffness.






Similar content being viewed by others
Data availability
Data will be provided upon request.
References
Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B (2012) The mechanical memory of lung myofibroblasts. Integr Biol (Camb) 4:410–421. https://doi.org/10.1039/c2ib00149g
Chen CY, Caporizzo MA, Bedi K, Vite A, Bogush AI, Robison P, Heffler JG, Salomon AK, Kelly NA, Babu A, Morley MP, Margulies KB, Prosser BL (2018) Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat Med 24:1225–1233. https://doi.org/10.1038/s41591-018-0046-2
Chen CY, Salomon AK, Caporizzo MA, Curry S, Kelly NA, Bedi K, Bogush AI, Kramer E, Schlossarek S, Janiak P, Moutin M, Carrier L, Margulies KB, Prosser BL (2020) Depletion of vasohibin 1 speeds contraction and relaxation in failing human cardiomyocytes. Circ Res 127:e14–e27. https://doi.org/10.1161/CIRCRESAHA.119.315947
Corbin EA, Vite A, Peyster EG, Bhoopalam M, Brandimarto J, Wang X, Bennett AI, Clark AT, Cheng X, Turner KT, Musunuru K, Margulies KB (2019) Tunable and reversible substrate stiffness reveals a dynamic mechanosensitivity of cardiomyocytes. ACS Appl Mater Interfaces 11:20603–20614. https://doi.org/10.1021/acsami.9b02446
Doppler SA, Carvalho C, Lahm H, Deutsch M, Dressen M, Puluca N, Lange R, Krane M (2017) Cardiac fibroblasts: more than mechanical support. J Thorac Dis 9:S36–S51. https://doi.org/10.21037/jtd.2017.03.122
Even-Ram S, Artym V, Yamada KM (2006) Matrix control of stem cell fate. Cell 126:645–647. https://doi.org/10.1016/j.cell.2006.08.008
Eyckmans J, Chen CS (2014) Stem cell differentiation: sticky mechanical memory. Nat Mater 13:542–543. https://doi.org/10.1038/nmat3989
Haque ZK, Wang D (2017) How cardiomyocytes sense pathophysiological stresses for cardiac remodeling. Cell Mol Life Sci 74:983–1000. https://doi.org/10.1007/s00018-016-2373-0
Heo S, Thorpe SD, Driscoll TP, Duncan RL, Lee DA, Mauck RL (2015) Biophysical regulation of chromatin architecture instills a mechanical memory in mesenchymal stem cells. Sci Rep 5:16895. https://doi.org/10.1038/srep16895
Kessler D, Dethlefsen S, Haase I, Plomann M, Hirche F, Krieg T, Eckes B (2001) Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J Biol Chem 276:36575–36585. https://doi.org/10.1074/jbc.M101602200
Martino F, Perestrelo AR, Vinarsky V, Pagliari S, Forte G (2018) Cellular mechanotransduction: from tension to function. Front Physiol 9:824. https://doi.org/10.3389/fphys.2018.00824
Mitsui W, Tamura K, Mizutani T, Haga H, Kawabata K (2009) Mechanical response of single myoblasts to various stretching patterns visualized by scanning probe microscopy. Arch Histol Cytol 72:227–234. https://doi.org/10.1679/aohc.72.227
Mostert D, Groenen B, Klouda L, Passier R, Goumans M, Kurniawan NA, Bouten CVC (2022) Human pluripotent stem cell-derived cardiomyocytes align under cyclic strain when guided by cardiac fibroblasts. APL Bioeng 6:046108. https://doi.org/10.1063/5.0108914
Munch J, Abdelilah-Seyfried S (2021) Sensing and responding of cardiomyocytes to changes of tissue stiffness in the diseased heart. Front Cell Dev Biol 9:642840. https://doi.org/10.3389/fcell.2021.642840
Nasrollahi S, Walter C, Loza AJ, Schimizzi GV, Longmore GD, Pathak A (2017) Past matrix stiffness primes epithelial cells and regulates their future collective migration through a mechanical memory. Biomaterials 146:146–155. https://doi.org/10.1016/j.biomaterials.2017.09.012
Ohashi K, Fujiwara S, Mizuno K (2017) Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J Biochem 161:245–254. https://doi.org/10.1093/jb/mvw082
Parker KK, Ingber DE (2007) Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Philos Trans R Soc Lond B Biol Sci 362:1267–1279. https://doi.org/10.1098/rstb.2007.2114
Robison P, Caporizzo MA, Ahmadzadeh H, Bogush AI, Chen CY, Margulies KB, Shenoy VB, Prosser BL (2016) Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science 352:aff0659. https://doi.org/10.1126/science.aaf0659
Saucerman JJ, Tan PM, Buchholz KS, McCulloch AD, Omens JH (2019) Mechanical regulation of gene expression in cardiac myocytes and fibroblasts. Nat Rev Cardiol 16:361–378. https://doi.org/10.1038/s41569-019-0155-8
Scott AK, Rafuse M, Neu CP (2023) Mechanically induced alterations in chromatin architecture guide the balance between cell plasticity and mechanical memory. Front Cell Dev Biol 11:1084759. https://doi.org/10.3389/fcell.2023.1084759
Spagnol ST, Dahl KN (2016) Spatially resolved quantification of chromatin condensation through differential local rheology in cell nuclei fluorescence lifetime imaging. PLoS ONE 11:e0146244. https://doi.org/10.1371/journal.pone.0146244
Sutton MG, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101:2981–2988. https://doi.org/10.1161/01.cir.101.25.2981
Tsuda T (2021) Clinical Assessment of ventricular wall stress in understanding compensatory hypertrophic response and maladaptive ventricular remodeling. J Cardiovasc Dev Dis 8:122. https://doi.org/10.3390/jcdd8100122
Vite A, Caporizzo MA, Corbin EA, Brandimarto J, McAfee Q, Livingston CE, Prosser BL, Margulies KB (2022) Extracellular stiffness induces contractile dysfunction in adult cardiomyocytes via cell-autonomous and microtubule-dependent mechanisms. Basic Res Cardiol 117:41–45. https://doi.org/10.1007/s00395-022-00952-5
Voorhees AP, Han H (2015) Biomechanics of cardiac function. Compr Physiol 5:1623–1644. https://doi.org/10.1002/cphy.c140070
Walker CJ, Crocini C, Ramirez D, Killaars AR, Grim JC, Aguado BA, Clark K, Allen MA, Dowell RD, Leinwand LA, Anseth KS (2021) Nuclear mechanosensing drives chromatin remodelling in persistently activated fibroblasts. Nat Biomed Eng 5:1485–1499. https://doi.org/10.1038/s41551-021-00709-w
Yang C, Tibbitt MW, Basta L, Anseth KS (2014) Mechanical memory and dosing influence stem cell fate. Nat Mater 13:645–652. https://doi.org/10.1038/nmat3889
Zhou C, Duan M, Guo D, Du X, Zhang D, **e J (2022) Microenvironmental stiffness mediates cytoskeleton re-organization in chondrocytes through laminin-FAK mechanotransduction. Int J Oral Sci 14:15–25. https://doi.org/10.1038/s41368-022-00165-5
Funding
This research was supported by funding from NIH/NHLBI R01-HL149891-01 to K.B.M, a Leducq Foundation award TNE ID#: 673168 to K.B.M., and an AHA postdoctoral fellowship award #1030560 to N.B.D.
Author information
Authors and Affiliations
Contributions
NB and KBM developed the research strategy, NB and AV participated in the design of the experiments. NB performed all experiments. NB and KBM participated in the writing of the manuscript. All authors participated in the review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Margulies reports significant financial interest: invention disclosure/patent, inventor: US patent application No.15/959,181 USA 2018, composition and methods for improving heart function and treating heart failure.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouhrira, N., Vite, A. & Margulies, K.B. Distinct cytoskeletal regulators of mechanical memory in cardiac fibroblasts and cardiomyocytes. Basic Res Cardiol 119, 277–289 (2024). https://doi.org/10.1007/s00395-023-01030-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-023-01030-0