Abstract
Purpose
Gut microbiota has been reported to contribute to either prevent or promote colorectal cancer (CRC), and treatment with probiotics might be a promising intervention method. The present study aimed to evaluate the potential anti-CRC effects of Lactobacillus coryniformis MXJ32 on a colitis-associated (CA)-CRC mouse model.
Methods
The CA-CRC mouse model was induced by a single intraperitoneal injection of 10 mg/kg azoxymethane and followed by three 7-day cycles of 2% dextran sulfate sodium in drinking water with a 14-day recovery period. Mice were supplemented with L. coryniformis MXJ32 by oral gavage (1 × 109 CFU/day/mouse). The CA-CRC attenuating effects of this probiotic were assessed via intestinal barrier integrity, inflammation, and gut microenvironment.
Results
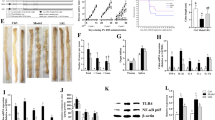
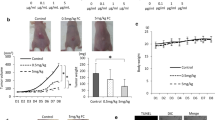
Treatment with L. coryniformis MXJ32 could significantly inhibit the total number of tumors and the average tumor diameter. This probiotic administration prevented the damage of intestinal barrier function by enhancing the expression of tight junction proteins (Occludin, Claudin-1, and ZO-1) and recovering the loss of goblet cells. Moreover, L. coryniformis MXJ32 alleviated intestinal inflammation via down-regulating the expression of inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-γ, and IL-17a) and chemokines (Cxcl1, Cxcl2, Cxcl3, Cxcl5, and Ccl7). In addition, L. coryniformis MXJ32 supplementation increased the abundance of some beneficial bacteria (such as SCFAs-producing bacteria, Lactobacillus, Bifidobacterium, Akkermansia, and Faecalibaculum) and decreased the abundance of some harmful bacteria (such as pro-inflammatory bacteria, Desulfovibrio and Helicobacter), which in turn attenuated the overexpression of inflammation.
Conclusion
Lactobacillus coryniformis MXJ32 could effectively ameliorate CA-CRC via regulating intestinal microenvironment, alleviating inflammation, and intestinal barrier damage, which further suggested that L. coryniformis MXJ32 could be considered as a functional food ingredient for the alleviation of CA-CRC.
Graphic abstract









Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- AOM:
-
Azoxymethane
- CA-CRC:
-
Colitis-associated colorectal cancer
- CFU:
-
Colony forming unit
- CXCR:
-
C-X-C motif receptor
- DAI:
-
Disease activity index
- DSS:
-
Dextran sulfate sodium
- FITC:
-
Fluorescein isothiocyanate
- H&E:
-
Hematoxylin and eosin
- IL:
-
Interleukin
- LDA:
-
Linear discriminant analysis
- LEfSe:
-
Linear discriminant analysis effect size
- LPS:
-
Lipopolysaccharides
- MUC:
-
Mucin
- OUT:
-
Operational taxonomic unit
- PCoA:
-
Principal coordinates analysis
- SCFA:
-
Short-chain fatty acid
- TFF:
-
Trefoil factor
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- UPGMA:
-
Unweighted pair group method with arithmetic mean
- ZO-1:
-
Tight junction protein-1
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Yin L, Meng Z, Zhang YX, Hu KK, Chen WY, Han KB, Wu BY, You R, Li CH, ** Y, Guan YQ (2018) Bacillus spore-based oral carriers loading curcumin for the therapy of colon cancer. J Control Release 271:31–44. https://doi.org/10.1016/j.jconrel.2017.12.013
Yue YC, Ye K, Lu J, Wang XY, Zhang SW, Liu L, Yang BY, Nassar K, Xu XX, Pang XY, Lv JP (2020) Probiotic strain Lactobacillus plantarum YYC-3 prevents colon cancer in mice by regulating the tumour microenvironment. Biomed Pharmacother 127:1–8. https://doi.org/10.1016/j.biopha.2020.110159
O’Brien JM (2000) Environmental and heritable factors in the causation of cancer: analyses of cohorts of twins from Sweden, Denmark, and Finland, by P. Lichtenstein, N.V. Holm, P.K. Verkasalo, A. Iliadou, J. Kaprio, M. Koskenvuo, E. Pukkala, A. Skytthe, and K. Hemminki. N Engl J Med 343:78-84, 2000. Surv Ophthalmol 45(2):167–168. https://doi.org/10.1016/s0039-6257(00)00165-x
Czene K, Lichtenstein P, Hemminki K (2002) Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish family-cancer database. Int J Cancer 99(2):260–266. https://doi.org/10.1002/ijc.10332
Wong SH, Yu J (2019) Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol 16(11):690–704. https://doi.org/10.1038/s41575-019-0209-8
Donaldson GP, Lee SM, Mazmanian SK (2016) Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 14(1):20–32. https://doi.org/10.1038/nrmicro3552
Tian Y, Zhou Y, Huang SS, Li J, Zhao K, Li XH, Wen XC, Li XA (2019) Fecal microbiota transplantation for ulcerative colitis: a prospective clinical study. BMC Gastroenterol 19:12. https://doi.org/10.1186/s12876-019-1010-4
Uronis JM, Muhlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C (2009) Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS ONE 4(6):e6026. https://doi.org/10.1371/journal.pone.0006026
Li YH, Wang S, Sun Y, Xu WQ, Zheng HN, Wang Y, Tang Y, Gao XW, Song C, Long Y, Liu JY, Liu L, Mei QB (2020) Apple polysaccharide protects ICR mice against colitis associated colorectal cancer through the regulation of microbial dysbiosis. Carbohydr Polym 230:10. https://doi.org/10.1016/j.carbpol.2019.115726
Jacouton E, Chain F, Sokol H, Langella P, Bermudez-Humaran LG (2017) Probiotic strain Lactobacillus casei BL23 prevents colitis-associated colorectal cancer. Front Immunol 8:10. https://doi.org/10.3389/fimmu.2017.01553
Silveira DSC, Veronez LC, Lopes LC, Anatriello E, Brunaldi MO, Pereira-da-Silva G (2020) Lactobacillus bulgaricus inhibits colitis-associated cancer via a negative regulation of intestinal inflammation in azoxymethane/dextran sodium sulfate model. World J Gastroenterol 26(43):6782–6794. https://doi.org/10.3748/wjg.v26.i43.6782
Eslami M, Yousefi B, Kokhaei P, Hemati M, Nejad ZR, Arabkari V, Namdar A (2019) Importance of probiotics in the prevention and treatment of colorectal cancer. J Cell Physiol 234(10):17127–17143. https://doi.org/10.1002/jcp.28473
Lu X, Yi LH, Dang J, Dang Y, Liu BF (2014) Purification of novel bacteriocin produced by Lactobacillus coryniformis MXJ32 for inhibiting bacterial foodborne pathogens including antibiotic-resistant microorganisms. Food Control 46:264–271. https://doi.org/10.1016/j.foodcont.2014.05.028
Wang T, Sun H, Chen J, Luo L, Gu Y, Wang X, Shan Y, Yi Y, Liu B, Zhou Y, Lu X (2021) Anti-adhesion effects of Lactobacillus strains on Caco-2 cells against Escherichia Coli and their application in ameliorating the symptoms of dextran sulfate sodium-induced colitis in mice. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-021-09774-8
Chen WG, Liu FL, Ling ZX, Tong XJ, **ang C (2012) Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 7(6):9. https://doi.org/10.1371/journal.pone.0039743
Yao DB, Dong M, Dai CL, Wu SD (2019) Inflammation and inflammatory cytokine contribute to the initiation and development of ulcerative colitis and its associated cancer. Inflamm Bowel Dis 25(10):1595–1602. https://doi.org/10.1093/ibd/izz149
Sun J, Chen H, Kan J, Gou YR, Liu J, Zhang X, Wu XN, Tang SX, Sun R, Qian CL, Zhang NF, Niu FX, ** CH (2020) Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int J Biol Macromol 153:708–722. https://doi.org/10.1016/j.ijbiomac.2020.03.053
Yang HX, Wang WC, Romano KA, Gu M, Sanidad KZ, Kim D, Yang J, Schmidt B, Panigrahy D, Pei RS, Martin DA, Ozay EI, Wang YX, Song MY, Bolling BW, **ao H, Minter LM, Yang GY, Liu ZH, Rey FE, Zhang GD (2018) A common antimicrobial additive increases colonic inflammation and colitis-associated colon tumorigenesis in mice. Sci Transl Med 10(443):10. https://doi.org/10.1126/scitranslmed.aan4116
**e F, Zhang H, Zheng C, Shen XF (2020) Costunolide improved dextran sulfate sodium-induced acute ulcerative colitis in mice through NF-kappa B, STAT1/3, and Akt signaling pathways. Int Immunopharmacol 84:10. https://doi.org/10.1016/j.intimp.2020.106567
Wang T, Yan H, Lu YY, Li X, Wang X, Shan YY, Yi YL, Liu BF, Zhou Y, Lu X (2020) Anti-obesity effect of Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047 on high-fat and high-fructose diet mice base on inflammatory response alleviation and gut microbiota regulation. Eur J Nutr 59(6):2709–2728. https://doi.org/10.1007/s00394-019-02117-y
Song H, Wang WY, Shen B, Jia H, Hou ZY, Chen P, Sun YW (2018) Pretreatment with probiotic Bifico ameliorates colitis-associated cancer in mice: transcriptome and gut flora profiling. Cancer Sci 109(3):666–677. https://doi.org/10.1111/cas.13497
Cammarota R, Bertolini V, Pennesi G, Bucci EO, Gottardi O, Garlanda C, Laghi L, Barberis MC, Sessa F, Noonan DM, Albini A (2010) The tumor microenvironment of colorectal cancer: Stromal TLR-4 expression as a potential prognostic marker. J Transl Med 8:16. https://doi.org/10.1186/1479-5876-8-112
van Staa TP, Card T, Logan RF, Leufkens HG (2005) 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut 54(11):1573–1578. https://doi.org/10.1136/gut.2005.070896
Flores BM, O’Connor A, Moss AC (2017) Impact of mucosal inflammation on risk of colorectal neoplasia in patients with ulcerative colitis: a systematic review and meta-analysis. Gastrointest Endosc 86(6):1006-1011.e1008. https://doi.org/10.1016/j.gie.2017.07.028
Wang DZ, DuBois RN, Richmond A (2009) The role of chemokines in intestinal inflammation and cancer. Curr Opin Pharmacol 9(6):688–696. https://doi.org/10.1016/j.coph.2009.08.003
Wang K, ** X, Li Q, Sawaya ACHF, Le Leu RK, Conlon MA, Wu L, Hu F (2018) Propolis from different geographic origins decreases intestinal inflammation and Bacteroides spp. populations in a model of DSS-induced colitis. Mol Nutr Food Res 62(17):1800080. https://doi.org/10.1002/mnfr.201800080
Khazaie K, Zadeh M, Khan MW, Bere P, Gounari F, Dennis K, Blatner NR, Owen JL, Klaenhammer TR, Mohamadzadeh M (2012) Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci USA 109(26):10462–10469. https://doi.org/10.1073/pnas.1207230109
Soroosh A, Rankin CR, Polytarchou C, Lokhandwala ZA, Patel A, Chang L, Pothoulakis C, Iliopoulos D, Padua DM (2019) miR-24 Is elevated in ulcerative colitis patients and regulates intestinal epithelial barrier function. Am J Pathol 189(9):1763–1774. https://doi.org/10.1016/j.ajpath.2019.05.018
Katinios G, Casado-Bedmar M, Walter SA, Vicario M, Gonzalez-Castro AM, Bednarska O, Soderholm JD, Hjortswang H, Keita AV (2020) Increased colonic epithelial permeability and mucosal eosinophilia in ulcerative colitis in remission compared with irritable bowel syndrome and health. Inflamm Bowel Dis 26(7):974–984. https://doi.org/10.1093/ibd/izz328
Robrahn L, Jiao L, Cramer T (2020) Barrier integrity and chronic inflammation mediated by HIF-1 impact on intestinal tumorigenesis. Cancer Lett 490:186–192. https://doi.org/10.1016/j.canlet.2020.07.002
Sun J, Kato I (2016) Gut microbiota, inflammation and colorectal cancer. Genes Dis 3(2):130–143. https://doi.org/10.1016/j.gendis.2016.03.004
Zeisel MB, Dhawan P, Baumert TF (2019) Tight junction proteins in gastrointestinal and liver disease. Gut 68(3):547–561. https://doi.org/10.1136/gutjnl-2018-316906
Kang MS, Martin A (2017) Microbiome and colorectal cancer: unraveling host-microbiota interactions in colitis-associated colorectal cancer development. Semin Immunol 32(C):3–13. https://doi.org/10.1016/j.smim.2017.04.003
Gao ZG, Guo BM, Gao RY, Zhu QC, Wu W, Qin HL (2015) Probiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancer. Mol Med Report 12(4):6119–6127. https://doi.org/10.3892/mmr.2015.4124
Wierzbicka A, Mankowska-Wierzbicka D, Mardas M, Stelmach-Mardas M (2021) Role of probiotics in modulating human gut microbiota populations and activities in patients with colorectal cancer-a systematic review of clinical trials. Nutrients 13(4):13. https://doi.org/10.3390/nu13041160
Peck BCE, Mah AT, Pitman WA, Ding SL, Lund PK, Sethupathy P (2017) Functional transcriptomics in diverse intestinal epithelial cell types reveals robust microRNA sensitivity in intestinal stem cells to microbial status. J Biol Chem 292(7):2586–2600. https://doi.org/10.1074/jbc.M116.770099
Oh NS, Lee JY, Kim YT, Kim SH, Lee JH (2020) Cancer-protective effect of a synbiotic combination between Lactobacillus gasseri 505 and a Cudrania tricuspidata leaf extract on colitis-associated colorectal cancer. Gut Microbes 12(1):20. https://doi.org/10.1080/19490976.2020.1785803
Wang Q, Wang KC, Wu WR, Lv LX, Bian XY, Yang LY, Wang QQ, Li YT, Ye JH, Fang DQ, Wu JJ, Jiang XW, **e JJ, Lu YM, Li LJ (2020) Administration of Bifidobacterium bifidum CGMCC 15068 modulates gut microbiota and metabolome in azoxymethane (AOM)/dextran sulphate sodium (DSS)-induced colitis-associated colon cancer (CAC) in mice. Appl Microbiol Biotechnol 104(13):5915–5928. https://doi.org/10.1007/s00253-020-10621-z
Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, Subbaramaiah K, Cooper HS, Itzkowitz SH, Abreu MT (2007) Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology 133(6):1869–1881. https://doi.org/10.1053/j.gastro.2007.09.008
De Simone V, Franze E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald TT, Pallone F, Monteleone G, Stolfi C (2015) Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 34(27):3493–3503. https://doi.org/10.1038/onc.2014.286
Zagato E, Pozzi C, Bertocchi A, Schioppa T, Saccheri F, Guglietta S, Fosso B, Melocchi L, Nizzoli G, Troisi J, Marzano M, Oresta B, Spadoni I, Atarashi K, Carloni S, Arioli S, Fornasa G, Asnicar F, Segata N, Guglielmetti S, Honda K, Pesole G, Vermi W, Penna G, Rescigno M (2020) Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat Microbiol 5(3):511–524. https://doi.org/10.1038/s41564-019-0649-5
Gomes SD, Oliveira CS, Azevedo-Silva J, Casanova MR, Barreto J, Pereira H, Chaves SR, Rodrigues LR, Casal M, Corte-Real M, Baltazar F, Preto A (2020) The role of diet related short-chain fatty acids in colorectal cancer metabolism and survival: prevention and therapeutic implications. Curr Med Chem 27(24):4087–4108. https://doi.org/10.2174/0929867325666180530102050
Yao Y, Cai X, Fei W, Ye Y, Zhao M, Zheng C (2020) The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2020.1854675
Li M, van Esch B, Wagenaar GTM, Garssen J, Folkerts G, Henricks PAJ (2018) Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol 831:52–59. https://doi.org/10.1016/j.ejphar.2018.05.003
Chang PV, Hao LM, Offermanns S, Medzhitov R (2014) The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA 111(6):2247–2252. https://doi.org/10.1073/pnas.1322269111
Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi HD, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40(1):128–139. https://doi.org/10.1016/j.immuni.2013.12.007
Acknowledgements
The author thanks the financial support of Post-doctoral Start-up funding (2018) of Northwest A&F University (Z109021804) and National Natural Science Foundation of China (Grant no. 31972043 and 32001652).
Author information
Authors and Affiliations
Contributions
TW, BL, XW, and XL designed the study and wrote the manuscript; TW and LZ performed the experiments; PW and YL analyzed the data; TW, GW, and YS interpreted the results of experiments; YY and YZ prepared figures. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Ethics declarations
All animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals: Eighth Edition, ISBN-10: 0-309-15396-4, and were approved by the Animal Ethics Committee of **’an Jiaotong University (Permission No. SCXK 2018-001).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, T., Zhang, L., Wang, P. et al. Lactobacillus coryniformis MXJ32 administration ameliorates azoxymethane/dextran sulfate sodium-induced colitis-associated colorectal cancer via resha** intestinal microenvironment and alleviating inflammatory response. Eur J Nutr 61, 85–99 (2022). https://doi.org/10.1007/s00394-021-02627-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02627-8